Bleomycin modulates amyloid aggregation in β-amyloid and hIAPP
- PMID: 35518630
- PMCID: PMC9055351
- DOI: 10.1039/d0ra04949b
Bleomycin modulates amyloid aggregation in β-amyloid and hIAPP
Abstract
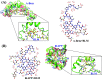
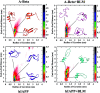
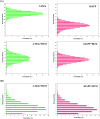
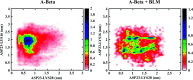
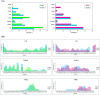
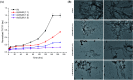
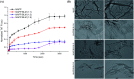
Aberrant misfolding and amyloid aggregation, which result in amyloid fibrils, are frequent and critical pathological incidents in various neurodegenerative disorders. Multiple drugs or inhibitors have been investigated to avert amyloid aggregation in individual peptides, exhibiting sequence-dependent inhibition mechanisms. Establishing or inventing inhibitors capable of preventing amyloid aggregation in a wide variety of amyloid peptides is quite a daunting task. Bleomycin (BLM), a complex glycopeptide, has been widely used as an antibiotic and antitumor drug due to its ability to inhibit DNA metabolism, and as an antineoplastic, especially for solid tumors. In this study, we investigated the dual inhibitory effects of BLM on Aβ aggregation, associated with Alzheimer's disease and hIAPP, which is linked to type 2 diabetes, using both computational and experimental techniques. Combined results from drug repurposing and replica exchange molecular dynamics simulations demonstrate that BLM binds to the β-sheet region considered a hotspot for amyloid fibrils of Aβ and hIAPP. BLM was also found to be involved in β-sheet destabilization and, ultimately, in its reduction. Further, experimental validation through in vitro amyloid aggregation assays was obtained wherein the fibrillar load was decreased for the BLM-treated Aβ and hIAPP peptides in comparison to controls. For the first time, this study shows that BLM is a dual inhibitor of Aβ and hIAPP amyloid aggregation. In the future, the conformational optimization and processing of BLM may help develop various efficient sequence-dependent inhibitors against amyloid aggregation in various amyloid peptides.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
No conflict of interest was declared by the authors regarding the content of this research article.
Figures


References
-
- Dobson C. M. Knowles T. P. Vendruscolo M. The amyloid phenomenon and its significance in biology and medicine. Cold Spring Harbor Perspect. Biol. 2019:a033878.
LinkOut - more resources
Full Text Sources

