Transient Receptor Potential Vanilloid 1 Function at Central Synapses in Health and Disease
- PMID: 35518644
- PMCID: PMC9062234
- DOI: 10.3389/fncel.2022.864828
Transient Receptor Potential Vanilloid 1 Function at Central Synapses in Health and Disease
Abstract
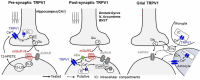
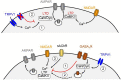
The transient receptor potential vanilloid 1 (TRPV1), a ligand-gated nonselective cation channel, is well known for mediating heat and pain sensation in the periphery. Increasing evidence suggests that TRPV1 is also expressed at various central synapses, where it plays a role in different types of activity-dependent synaptic changes. Although its precise localizations remain a matter of debate, TRPV1 has been shown to modulate both neurotransmitter release at presynaptic terminals and synaptic efficacy in postsynaptic compartments. In addition to being required in these forms of synaptic plasticity, TRPV1 can also modify the inducibility of other types of plasticity. Here, we highlight current evidence of the potential roles for TRPV1 in regulating synaptic function in various brain regions, with an emphasis on principal mechanisms underlying TRPV1-mediated synaptic plasticity and metaplasticity. Finally, we discuss the putative contributions of TRPV1 in diverse brain disorders in order to expedite the development of next-generation therapeutic treatments.
Keywords: brain; endocannabinoids; neurotransmision; synaptic function; vanilloid receptor.
Copyright © 2022 Meza, Ancatén-González, Chiu and Chávez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The transient receptor potential vanilloid-1 is localized at excitatory synapses in the mouse dentate gyrus.Brain Struct Funct. 2015 Mar;220(2):1187-94. doi: 10.1007/s00429-014-0711-2. Epub 2014 Feb 1. Brain Struct Funct. 2015. PMID: 24487914
-
The Absence of the Transient Receptor Potential Vanilloid 1 Directly Impacts on the Expression and Localization of the Endocannabinoid System in the Mouse Hippocampus.Front Neuroanat. 2021 Feb 22;15:645940. doi: 10.3389/fnana.2021.645940. eCollection 2021. Front Neuroanat. 2021. PMID: 33692673 Free PMC article.
-
Ultrastructural analysis of the synaptic connectivity of TRPV1-expressing primary afferent terminals in the rat trigeminal caudal nucleus.J Comp Neurol. 2010 Oct 15;518(20):4134-46. doi: 10.1002/cne.22369. J Comp Neurol. 2010. PMID: 20878780
-
TRPV1 and Endocannabinoids: Emerging Molecular Signals that Modulate Mammalian Vision.Cells. 2014 Sep 12;3(3):914-38. doi: 10.3390/cells3030914. Cells. 2014. PMID: 25222270 Free PMC article. Review.
-
TRPV1 and synaptic transmission.Curr Pharm Biotechnol. 2011 Jan 1;12(1):95-101. doi: 10.2174/138920111793937925. Curr Pharm Biotechnol. 2011. PMID: 20932254 Review.
Cited by
-
TRPV1 Channels in the Central Nervous System as Drug Targets.Pharmaceuticals (Basel). 2024 Jun 7;17(6):756. doi: 10.3390/ph17060756. Pharmaceuticals (Basel). 2024. PMID: 38931423 Free PMC article. Review.
-
Pharmacologically targeting transient receptor potential channels for seizures and epilepsy: Emerging preclinical evidence of druggability.Pharmacol Ther. 2023 Apr;244:108384. doi: 10.1016/j.pharmthera.2023.108384. Epub 2023 Mar 16. Pharmacol Ther. 2023. PMID: 36933703 Free PMC article. Review.
-
URB937 Prevents the Development of Mechanical Allodynia in Male Rats with Trigeminal Neuralgia.Pharmaceuticals (Basel). 2023 Nov 18;16(11):1626. doi: 10.3390/ph16111626. Pharmaceuticals (Basel). 2023. PMID: 38004491 Free PMC article.
-
"ThermoTRP" Channel Expression in Cancers: Implications for Diagnosis and Prognosis (Practical Approach by a Pathologist).Int J Mol Sci. 2023 May 22;24(10):9098. doi: 10.3390/ijms24109098. Int J Mol Sci. 2023. PMID: 37240443 Free PMC article. Review.
-
Magnetothermal-based non-invasive focused magnetic stimulation for functional recovery in chronic stroke treatment.Sci Rep. 2023 Mar 27;13(1):4988. doi: 10.1038/s41598-023-31979-w. Sci Rep. 2023. PMID: 36973390 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

