Fully automated peptide radiolabeling from [18F]fluoride
- PMID: 35518701
- PMCID: PMC9061836
- DOI: 10.1039/c8ra10541c
Fully automated peptide radiolabeling from [18F]fluoride
Abstract
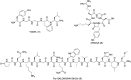
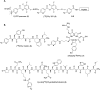
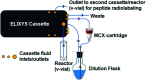
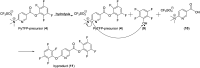
The biological properties of receptor-targeted peptides have made them popular diagnostic imaging and therapeutic agents. Typically, the synthesis of fluorine-18 radiolabeled receptor-targeted peptides for positron emission tomography (PET) imaging is a time consuming, complex, multi-step synthetic process that is highly variable based on the peptide. The complexity associated with the radiolabeling route and lack of robust automated protocols can hinder translation into the clinic. A fully automated batch production to radiolabel three peptides (YGGFL, cRGDyK, and Pyr-QKLGNQWAVGHLM) from fluorine-18 using the ELIXYS FLEX/CHEM® radiosynthesizer in a two-step process is described. First, the prosthetic group, 6-[18F]fluoronicotinyl-2,3,5,6-tetrafluorophenyl ester ([18F]FPy-TFP) was synthesized and subsequently attached to the peptide. The [18F]FPy-peptides were synthesized in 13-26% decay corrected yields from fluorine-18 with high molar activity 1-5 Ci μmol-1 and radiochemical purity of >99% in an overall synthesis time of 97 ± 3 minutes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Drs Drake and Moore are employees and owners of SOFIE Co.
Figures

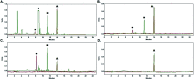
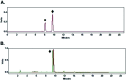
Similar articles
-
Automated Synthesis of Fluorine-18 Labeled CXCR4 Ligand via the Conjugation with Nicotinic Acid N-Hydroxysuccinimide Ester (6-[18F]SFPy).Molecules. 2020 Aug 27;25(17):3924. doi: 10.3390/molecules25173924. Molecules. 2020. PMID: 32867358 Free PMC article.
-
N,N,N-Trimethyl-5-[(2,3,5,6-tetrafluorophenoxy)carbonyl]pyridin-2-aminium trifluoromethanesulfonate a precursor for the synthesis of 2,3,5,6-tetrafluorophenyl 6-[18F]-fluoronicotinate.Acta Crystallogr C Struct Chem. 2018 May 1;74(Pt 5):604-607. doi: 10.1107/S2053229618005430. Epub 2018 Apr 18. Acta Crystallogr C Struct Chem. 2018. PMID: 29726470
-
Solid-phase synthesis and fluorine-18 radiolabeling of cycloRGDyK.Org Biomol Chem. 2016 Sep 21;14(37):8659-8663. doi: 10.1039/c6ob01636g. Org Biomol Chem. 2016. PMID: 27714190 Free PMC article.
-
An automated radiosynthesis of [18F]DPA-714 on a commercially available radiosynthesizer, Elixys Flex/Chem.Appl Radiat Isot. 2022 Feb;180:110032. doi: 10.1016/j.apradiso.2021.110032. Epub 2021 Nov 18. Appl Radiat Isot. 2022. PMID: 34871885 Free PMC article. Review.
-
Reaction of [18F]Fluoride at Heteroatoms and Metals for Imaging of Peptides and Proteins by Positron Emission Tomography.Front Chem. 2021 Jun 23;9:687678. doi: 10.3389/fchem.2021.687678. eCollection 2021. Front Chem. 2021. PMID: 34249861 Free PMC article. Review.
Cited by
-
Automated light-induced synthesis of 89Zr-radiolabeled antibodies for immuno-positron emission tomography.Sci Rep. 2022 Jan 13;12(1):668. doi: 10.1038/s41598-021-04626-5. Sci Rep. 2022. PMID: 35027637 Free PMC article.
-
Rapid cleavage of 6-[18F]fluoronicotinic acid prosthetic group governs BT12 glioblastoma xenograft uptake: implications for radiolabeling design of biomolecules.EJNMMI Radiopharm Chem. 2025 Jul 8;10(1):40. doi: 10.1186/s41181-025-00368-1. EJNMMI Radiopharm Chem. 2025. PMID: 40629197 Free PMC article.
-
Hetero-Diels-Alder and CuAAC Click Reactions for Fluorine-18 Labeling of Peptides: Automation and Comparative Study of the Two Methods.Molecules. 2024 Jul 5;29(13):3198. doi: 10.3390/molecules29133198. Molecules. 2024. PMID: 38999148 Free PMC article.
-
Automated radiofluorination of HER2 single domain antibody: the road towards the clinical translation of [18F]FB-HER2 sdAb.EJNMMI Radiopharm Chem. 2024 Nov 14;9(1):77. doi: 10.1186/s41181-024-00306-7. EJNMMI Radiopharm Chem. 2024. PMID: 39542993 Free PMC article.
-
A Fully Automated Synthesis of 14-(R,S)-[18F]fluoro-6-thia-heptadecanoic Acid ([18F]FTHA) on the Elixys Radiosynthesizer.Pharmaceuticals (Basel). 2024 Feb 29;17(3):318. doi: 10.3390/ph17030318. Pharmaceuticals (Basel). 2024. PMID: 38543104 Free PMC article.

