Chemoenzymatic Synthesis of Original Stilbene Dimers Possessing Wnt Inhibition Activity in Triple-Negative Breast Cancer Cells Using the Enzymatic Secretome of Botrytis cinerea Pers
- PMID: 35518712
- PMCID: PMC9062038
- DOI: 10.3389/fchem.2022.881298
Chemoenzymatic Synthesis of Original Stilbene Dimers Possessing Wnt Inhibition Activity in Triple-Negative Breast Cancer Cells Using the Enzymatic Secretome of Botrytis cinerea Pers
Abstract
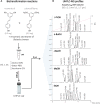
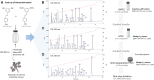
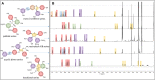
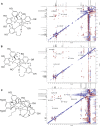
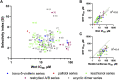
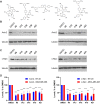
The Wnt signaling pathway controls multiple events during embryonic development of multicellular animals and is carcinogenic when aberrantly activated in adults. Breast cancer and triple-negative breast cancer (TNBC) in particular depend upon Wnt pathway overactivation. Despite this importance, no Wnt pathway-targeting drugs are currently available, which necessitates novel approaches to search for therapeutically relevant compounds targeting this oncogenic pathway. Stilbene analogs represent an under-explored field of therapeutic natural products research. In the present work, a library of complex stilbene derivatives was obtained through biotransformation of a mixture of resveratrol and pterostilbene using the enzymatic secretome of Botrytis cinerea. To improve the chemodiversity, the reactions were performed using i-PrOH, n-BuOH, i-BuOH, EtOH, or MeOH as cosolvents. Using this strategy, a series of 73 unusual derivatives was generated distributed among 6 scaffolds; 55 derivatives represent novel compounds. The structure of each compound isolated was determined by nuclear magnetic resonance and high-resolution mass spectrometry. The inhibitory activity of the isolated compounds against the oncogenic Wnt pathway was comprehensively quantified and correlated with their capacity to inhibit the growth of the cancer cells, leading to insights into structure-activity relationships of the derivatives. Finally, we have dissected mechanistic details of the stilbene derivatives activity within the pathway.
Keywords: Botrytis cinerea; Wnt inhibition; chemoenzymatic synthesis; dry load introduction; enzymatic secretome; high-resolution semi-preparative HPLC; stilbene dimers; triple negative breast cancer cells.
Copyright © 2022 Huber, Koval, Marcourt, Héritier, Schnee, Michellod, Scapozza, Katanaev, Wolfender, Gindro and Ferreira Queiroz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


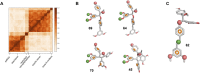
Similar articles
-
Chiral Separation of Stilbene Dimers Generated by Biotransformation for Absolute Configuration Determination and Antibacterial Evaluation.Front Chem. 2022 May 31;10:912396. doi: 10.3389/fchem.2022.912396. eCollection 2022. Front Chem. 2022. PMID: 35711965 Free PMC article.
-
Chemoenzymatic Synthesis of Complex Phenylpropanoid Derivatives by the Botrytis cinerea Secretome and Evaluation of Their Wnt Inhibition Activity.Front Plant Sci. 2022 Jan 13;12:805610. doi: 10.3389/fpls.2021.805610. eCollection 2021. Front Plant Sci. 2022. PMID: 35095976 Free PMC article.
-
Generation of Antifungal Stilbenes Using the Enzymatic Secretome of Botrytis cinerea.J Nat Prod. 2017 Apr 28;80(4):887-898. doi: 10.1021/acs.jnatprod.6b00760. Epub 2017 Mar 23. J Nat Prod. 2017. PMID: 28332842
-
Countering Triple Negative Breast Cancer via Impeding Wnt/β-Catenin Signaling, a Phytotherapeutic Approach.Plants (Basel). 2022 Aug 24;11(17):2191. doi: 10.3390/plants11172191. Plants (Basel). 2022. PMID: 36079579 Free PMC article. Review.
-
Role of microRNA/lncRNA Intertwined With the Wnt/β-Catenin Axis in Regulating the Pathogenesis of Triple-Negative Breast Cancer.Front Pharmacol. 2022 Jun 24;13:814971. doi: 10.3389/fphar.2022.814971. eCollection 2022. Front Pharmacol. 2022. PMID: 35814205 Free PMC article. Review.
Cited by
-
Advanced high-resolution chromatographic strategies for efficient isolation of natural products from complex biological matrices: from metabolite profiling to pure chemical entities.Phytochem Rev. 2024;23(5):1415-1442. doi: 10.1007/s11101-024-09928-w. Epub 2024 May 6. Phytochem Rev. 2024. PMID: 39574436 Free PMC article. Review.
-
Chiral Separation of Stilbene Dimers Generated by Biotransformation for Absolute Configuration Determination and Antibacterial Evaluation.Front Chem. 2022 May 31;10:912396. doi: 10.3389/fchem.2022.912396. eCollection 2022. Front Chem. 2022. PMID: 35711965 Free PMC article.
-
Discovery of anti-infective compounds against Mycobacterium marinum after biotransformation of simple natural stilbenes by a fungal secretome.Front Microbiol. 2024 Sep 17;15:1439814. doi: 10.3389/fmicb.2024.1439814. eCollection 2024. Front Microbiol. 2024. PMID: 39355425 Free PMC article.
-
Antiviral properties of trans-δ-viniferin derivatives against enveloped viruses.Biomed Pharmacother. 2023 Jul;163:114825. doi: 10.1016/j.biopha.2023.114825. Epub 2023 May 4. Biomed Pharmacother. 2023. PMID: 37148860 Free PMC article.
-
Study of phenoxy radical couplings using the enzymatic secretome of Botrytis cinerea.Front Chem. 2024 May 28;12:1390066. doi: 10.3389/fchem.2024.1390066. eCollection 2024. Front Chem. 2024. PMID: 38863677 Free PMC article.
References
-
- Adesanya S. A., Nia R., Martin M.-T., Boukamcha N., Montagnac A., Païs M. (1999). Stilbene Derivatives from Cissus quadrangularis . J. Nat. Prod. 62, 1694–1695. 10.1021/np9902744 - DOI
-
- An W. F., Germain A. R., Bishop J. A., Nag P. P., Metkar S., Ketterman J., et al. (2010). “Discovery of Potent and Highly Selective Inhibitors of GSK3b,” in Probe Reports from the NIH Molecular Libraries Program (Bethesda (MD: National Center for Biotechnology Information; ). - PubMed
LinkOut - more resources
Full Text Sources

