Fabrication of Guided Tissue Regeneration Membrane Using Lignin-Mediated ZnO Nanoparticles in Biopolymer Matrix for Antimicrobial Activity
- PMID: 35518713
- PMCID: PMC9063929
- DOI: 10.3389/fchem.2022.837858
Fabrication of Guided Tissue Regeneration Membrane Using Lignin-Mediated ZnO Nanoparticles in Biopolymer Matrix for Antimicrobial Activity
Abstract
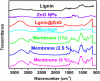
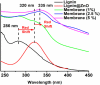
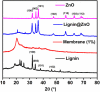
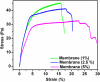
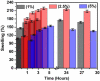
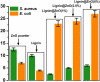
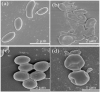
Periodontal disease is a common complication, and conventional periodontal surgery can lead to severe bleeding. Different membranes have been used for periodontal treatment with limitations, such as improper biodegradation, poor mechanical property, and no effective hemostatic property. Guided tissue regeneration (GTR) membranes favoring periodontal regeneration were prepared to overcome these shortcomings. The mucilage of the chia seed was extracted and utilized to prepare the guided tissue regeneration (GTR) membrane. Lignin having antibacterial properties was used to synthesize lignin-mediated ZnO nanoparticles (∼Lignin@ZnO) followed by characterization with analytical techniques like Fourier-transform infrared spectroscopy (FTIR), UV-visible spectroscopy, and scanning electron microscope (SEM). To fabricate the GTR membrane, extracted mucilage, Lignin@ZnO, and polyvinyl alcohol (PVA) were mixed in different ratios to obtain a thin film. The fabricated GTR membrane was evaluated using a dynamic fatigue analyzer for mechanical properties. Appropriate degradation rates were approved by degradability analysis in water for different intervals of time. The fabricated GTR membrane showed excellent antibacterial properties against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) bacterial species.
Keywords: GTR membrane; ZnO nanoparticles; antimicrobial activity; lignin; mucilage.
Copyright © 2022 Bilal, Niazi, Nadeem, Farid, Nazir, Akhter, Javed, Mohyuddin, Rauf, Ali, Naqvi, Muhammad, Elkaeed, Ibrahium, Awwad and Hassan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration.Dent Mater. 2015 Sep;31(9):1038-51. doi: 10.1016/j.dental.2015.06.004. Epub 2015 Jun 24. Dent Mater. 2015. PMID: 26116414 Free PMC article.
-
Recent progress in materials development and biological properties of GTR membranes for periodontal regeneration.Chem Biol Drug Des. 2021 Dec;98(6):1007-1024. doi: 10.1111/cbdd.13959. Epub 2021 Oct 11. Chem Biol Drug Des. 2021. PMID: 34581497 Review.
-
ZnO nanoparticles-modified polycaprolactone-gelatin membranes for guided/bone tissue regeneration, antibacterial and osteogenic differentiation properties.Biomed Phys Eng Express. 2023 Mar 10;9(3). doi: 10.1088/2057-1976/acbe47. Biomed Phys Eng Express. 2023. PMID: 36821850
-
Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane.Mater Sci Eng C Mater Biol Appl. 2020 Apr;109:110618. doi: 10.1016/j.msec.2019.110618. Epub 2020 Jan 3. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32228889
-
Recent advances in the development of GTR/GBR membranes for periodontal regeneration--a materials perspective.Dent Mater. 2012 Jul;28(7):703-21. doi: 10.1016/j.dental.2012.04.022. Epub 2012 May 14. Dent Mater. 2012. PMID: 22592164 Review.
Cited by
-
Nano-Based Drug Delivery Systems for Periodontal Tissue Regeneration.Pharmaceutics. 2022 Oct 21;14(10):2250. doi: 10.3390/pharmaceutics14102250. Pharmaceutics. 2022. PMID: 36297683 Free PMC article. Review.
-
High value valorization of lignin as environmental benign antimicrobial.Mater Today Bio. 2022 Dec 15;18:100520. doi: 10.1016/j.mtbio.2022.100520. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36590981 Free PMC article. Review.
-
Regeneration Membranes Loaded with Non-Antibiotic Anti-2 Microbials: A Review.Polymers (Basel). 2023 Dec 28;16(1):95. doi: 10.3390/polym16010095. Polymers (Basel). 2023. PMID: 38201760 Free PMC article. Review.
-
Nuclear Nanomedicines: Utilization of Radiolabelling Strategies, Drug Formulation, Delivery, and Regulatory Aspects for Disease Management.Curr Radiopharm. 2025;18(4):262-282. doi: 10.2174/0118744710373025250423042401. Curr Radiopharm. 2025. PMID: 40304320 Review.
-
Laser-Assisted Preparation of TiO2/Carbon/Ag Nanocomposite for Degradation of Organic Pollutants.Materials (Basel). 2024 Aug 20;17(16):4118. doi: 10.3390/ma17164118. Materials (Basel). 2024. PMID: 39203296 Free PMC article.
References
-
- Aadil K. R., Pandey N., Mussatto S. I., Jha H. (2019). Green Synthesis of Silver Nanoparticles Using acacia Lignin, Their Cytotoxicity, Catalytic, Metal Ion Sensing Capability and Antibacterial Activity. J. Environ. Chem. Eng. 7, 103296. 10.1016/j.jece.2019.103296 - DOI
-
- Abbasi B. H., Anjum S., Hano C. (2017). Differential Effects of In Vitro Cultures of Linum usitatissimum L. (Flax) on Biosynthesis, Stability, Antibacterial and Antileishmanial Activities of Zinc Oxide Nanoparticles: a Mechanistic Approach. RSC Adv. 7, 15931–15943. 10.1039/c7ra02070h - DOI
-
- Abdelaziz D., Hefnawy A., Al-Wakeel E., El-Fallal A., El-Sherbiny I. M. (2021). New Biodegradable Nanoparticles-In-Nanofibers Based Membranes for Guided Periodontal Tissue and Bone Regeneration with Enhanced Antibacterial Activity. J. Adv. Res. 28, 51–62. 10.1016/j.jare.2020.06.014 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

