The role of PMA in enhancing the surface acidity and catalytic activity of a bimetallic Cr-Mg-MOF and its applications for synthesis of coumarin and dihydropyrimidinone derivatives
- PMID: 35518723
- PMCID: PMC9054398
- DOI: 10.1039/d0ra03591b
The role of PMA in enhancing the surface acidity and catalytic activity of a bimetallic Cr-Mg-MOF and its applications for synthesis of coumarin and dihydropyrimidinone derivatives
Erratum in
-
Correction: The role of PMA in enhancing the surface acidity and catalytic activity of a bimetallic Cr-Mg-MOF and its applications for synthesis of coumarin and dihydropyrimidinone derivatives.RSC Adv. 2024 Mar 7;14(12):8016-8017. doi: 10.1039/d4ra90020k. eCollection 2024 Mar 6. RSC Adv. 2024. PMID: 38454948 Free PMC article.
Abstract
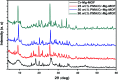
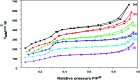
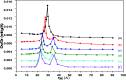
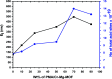
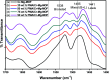
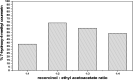
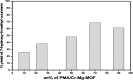
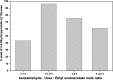
In the present study, a bimetallic Cr-Mg-MOF was successfully synthesized by the solvothermal method and then modified by loading different amounts of phosphomolybdic acid (PMA) using a simple wet impregnation technique. The morphological and structural properties of the prepared samples were investigated using X-ray diffraction, TEM, SEM, BET and FTIR spectroscopy. Importantly, Mg doping not only caused the Cr-Mg-MOF to have a higher surface area than MIL-101 (Cr) or MOF-74 (Mg), but the strategy of doping metal ions can be an effective way to improve the adsorption performance of MOFs. The surface acidity and the acid strength of the samples were determined using potentiometric titration and the FTIR of pyridine adsorption. The incorporation of PMA crystals gradually enhances both the surface acidity and the acid strength of the PMA/Cr-Mg-MOF catalysts up to 75 wt%. The catalytic performances of the prepared catalysts were tested in two acid-catalyzed organic transformations, namely, 7-hydroxy-4-methyl coumarin and 3,4-dihydropyrimidinone. In the two reactions, the catalytic activity attains the maximum value at 75 wt% PMA loading. The PMA catalysts supported on Cr-Mg-MOF are potentially promising heterogeneous catalysts for acid-catalyzed organic transformations in environmentally friendly processes, to replace the use of conventional homogeneous PMA catalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Hassan S. M. Ahmed A. I. Mannaa M. A. Journal of Science: Advanced Materials and Devices. 2019;4:400–412.
-
- Hassan S. M. Ahmed A. I. Mannaa M. A. Ceram. Int. 2018;44:6201–6211. doi: 10.1016/j.ceramint.2018.01.005. - DOI
-
- Mannaa M. A. Hassan S. M. Ahmed A. I. Int. J. Mod. Chem. 2018;10:69–79.
-
- Kimura M. Nakato T. Okuhara T. Appl. Catal., A. 1997;165:227–240. doi: 10.1016/S0926-860X(97)00204-4. - DOI
-
- Okuyama K. Chen X. Takata K. Odawara D. Suzuki T. Nakata S. Okuhara T. Appl. Catal., A. 2000;190:253–260. doi: 10.1016/S0926-860X(99)00321-X. - DOI
LinkOut - more resources
Full Text Sources

