Catalytic conversion of ethane to valuable products through non-oxidative dehydrogenation and dehydroaromatization
- PMID: 35518732
- PMCID: PMC9054567
- DOI: 10.1039/d0ra03365k
Catalytic conversion of ethane to valuable products through non-oxidative dehydrogenation and dehydroaromatization
Abstract
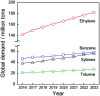
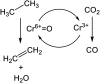
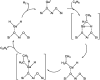
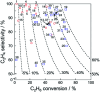
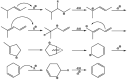
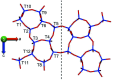
Chemical utilization of ethane to produce valuable chemicals has become especially attractive since the expanded utilization of shale gas in the United States and associated petroleum gas in the Middle East. Catalytic conversion to ethylene and aromatic hydrocarbons through non-oxidative dehydrogenation and dehydroaromatization of ethane (EDH and EDA) are potentially beneficial technologies because of their high selectivity to products. The former represents an attractive alternative to conventional thermal cracking of ethane. The latter can produce valuable aromatic hydrocarbons from a cheap feedstock. Nevertheless, further progress in catalytic science and technology is indispensable to implement these processes beneficially. This review summarizes progress that has been achieved with non-oxidative EDH and EDA in terms of the nature of active sites and reaction mechanisms. Briefly, platinum-, chromium- and gallium-based catalysts have been introduced mainly for EDH, including effects of carbon dioxide co-feeding. Efforts to use EDA have emphasized zinc-modified MFI zeolite catalysts. Finally, some avenues for development of catalytic science and technology for ethane conversion are summarized.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










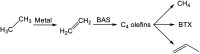
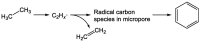


Similar articles
-
Discrimination of the Synergistic Effect of Different Zinc Active Sites with a Brønsted Acid in Zeolite for Dehydrogenation Cracking of n-Octane and Ethane Dehydroaromatization.Langmuir. 2024 Dec 31;40(52):27470-27480. doi: 10.1021/acs.langmuir.4c03769. Epub 2024 Dec 17. Langmuir. 2024. PMID: 39688097
-
Redefining the Role of Cobalt Oxide in Ethane Dehydroaromatization: Insights into Enhanced Catalytic Activity and Stability.ACS Appl Mater Interfaces. 2025 Jan 8;17(1):823-834. doi: 10.1021/acsami.4c13379. Epub 2024 Dec 25. ACS Appl Mater Interfaces. 2025. PMID: 39720877
-
Recent Advances on Gallium-Modified ZSM-5 for Conversion of Light Hydrocarbons.Molecules. 2021 Apr 13;26(8):2234. doi: 10.3390/molecules26082234. Molecules. 2021. PMID: 33924390 Free PMC article. Review.
-
Reactivity, Selectivity, and Stability of Zeolite-Based Catalysts for Methane Dehydroaromatization.Adv Mater. 2020 Nov;32(44):e2002565. doi: 10.1002/adma.202002565. Epub 2020 Jul 12. Adv Mater. 2020. PMID: 32656906 Review.
-
Coking-Resistant Iron Catalyst in Ethane Dehydrogenation Achieved through Siliceous Zeolite Modulation.J Am Chem Soc. 2020 Sep 23;142(38):16429-16436. doi: 10.1021/jacs.0c07792. Epub 2020 Sep 11. J Am Chem Soc. 2020. PMID: 32862644
Cited by
-
Catalytic selective ethane dehydrogenation at low-temperature with low coke formation.RSC Adv. 2022 Aug 30;12(38):24465-24470. doi: 10.1039/d2ra04401c. eCollection 2022 Aug 30. RSC Adv. 2022. PMID: 36128363 Free PMC article.
-
Zeolite Supported Pt for Depolymerization of Polyethylene by Induction Heating.Ind Eng Chem Res. 2023 May 24;62(22):8635-8643. doi: 10.1021/acs.iecr.2c04568. eCollection 2023 Jun 7. Ind Eng Chem Res. 2023. PMID: 37304911 Free PMC article.
-
Isolated Pt Atoms Stabilized by Ga2O3 Clusters Confined in ZSM-5 for Nonoxidative Activation of Ethane.JACS Au. 2024 Aug 26;4(9):3547-3557. doi: 10.1021/jacsau.4c00480. eCollection 2024 Sep 23. JACS Au. 2024. PMID: 39328764 Free PMC article.
-
Unraveling the Kinetics and Mechanism of Ethane Chlorination in the Gas Phase.Molecules. 2025 Apr 14;30(8):1756. doi: 10.3390/molecules30081756. Molecules. 2025. PMID: 40333805 Free PMC article.
-
Exploring Deactivation Reasons of Biomass-Based Phosphorus-Doped Carbon as a Metal-Free Catalyst in the Catalytic Dehydroaromatization of n-Heptane.Molecules. 2024 Mar 14;29(6):1288. doi: 10.3390/molecules29061288. Molecules. 2024. PMID: 38542924 Free PMC article.
References
-
- Boulamanti J. Moya A. Renewable Sustainable Energy Rev. 2017;68:1205. doi: 10.1016/j.rser.2016.02.021. - DOI
-
- Forecast of Global Supply and Demand Trends for Petrochemical Products (from 2010 to 2023), METI Japan, https://www.meti.go.jp/english/press/2019/1017_001.html, 2019, accessed on 20/5/2020
-
- Tian P. Wei Y. Ye M. Liu Z. ACS Catal. 2015;5:1922. doi: 10.1021/acscatal.5b00007. - DOI
Publication types
LinkOut - more resources
Full Text Sources

