Reducing toxic reactive carbonyl species in e-cigarette emissions: testing a harm-reduction strategy based on dicarbonyl trapping
- PMID: 35518766
- PMCID: PMC9054509
- DOI: 10.1039/d0ra02138e
Reducing toxic reactive carbonyl species in e-cigarette emissions: testing a harm-reduction strategy based on dicarbonyl trapping
Abstract
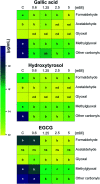
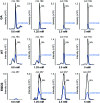
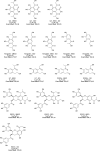
Reducing the concentration of reactive carbonyl species (RCS) in e-cigarette emissions represents a major goal to control their potentially harmful effects. Here, we adopted a novel strategy of trapping carbonyls present in e-cigarette emissions by adding polyphenols in e-liquid formulations. Our work showed that the addition of gallic acid, hydroxytyrosol and epigallocatechin gallate reduced the levels of carbonyls formed in the aerosols of vaped e-cigarettes, including formaldehyde, methylglyoxal and glyoxal. Liquid chromatography mass spectrometry analysis highlighted the formation of covalent adducts between aromatic rings and dicarbonyls in both e-liquids and vaped samples, suggesting that dicarbonyls were formed in the e-liquids as degradation products of propylene glycol and glycerol before vaping. Short-term cytotoxic analysis on two lung cellular models showed that dicarbonyl-polyphenol adducts are not cytotoxic, even though carbonyl trapping did not improve cell viability. Our work sheds lights on the ability of polyphenols to trap RCS in high carbonyl e-cigarette emissions, suggesting their potential value in commercial e-liquid formulations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors Bruna de Falco, W. Edryd Stephens and Alberto Fiore are associated with a patent pending on content relating to this manuscript.
Figures

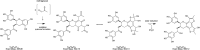

Similar articles
-
Tea polyphenol (-)-epigallocatechin-3-gallate: a new trapping agent of reactive dicarbonyl species.Chem Res Toxicol. 2007 Dec;20(12):1862-70. doi: 10.1021/tx700190s. Epub 2007 Nov 15. Chem Res Toxicol. 2007. PMID: 18001060
-
Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns.Int J Environ Res Public Health. 2020 Apr 17;17(8):2767. doi: 10.3390/ijerph17082767. Int J Environ Res Public Health. 2020. PMID: 32316435 Free PMC article.
-
A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents.PLoS One. 2017 Jan 11;12(1):e0169811. doi: 10.1371/journal.pone.0169811. eCollection 2017. PLoS One. 2017. PMID: 28076380 Free PMC article.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Reactive Carbonyl Species Scavenger: Epigallocatechin-3-Gallate.Foods. 2024 Mar 25;13(7):992. doi: 10.3390/foods13070992. Foods. 2024. PMID: 38611299 Free PMC article. Review.
Cited by
-
Patterns of Use of e-Cigarettes and Their Respiratory Effects: A Critical Umbrella Review.Tob Use Insights. 2025 Mar 11;18:1179173X251325421. doi: 10.1177/1179173X251325421. eCollection 2025. Tob Use Insights. 2025. PMID: 40078697 Free PMC article. Review.
-
Patterns of Use of e-Cigarettes and Their Respiratory Effects: Protocol for an Umbrella Review.JMIR Res Protoc. 2024 Sep 4;13:e60325. doi: 10.2196/60325. JMIR Res Protoc. 2024. PMID: 39230946 Free PMC article.
References
-
- McNeill A., Brose L. S., Calder R., Bauld L. and Robson D., A report commissioned by Public Health England, London, Public Health England, 2018, vol. 6
-
- Eriksen M., Mackay J. and Ross H., The tobacco atlas, American cancer society Inc., Atlanta, GA, USA, 2012
LinkOut - more resources
Full Text Sources
Other Literature Sources

