Stress stability study of simeprevir, a hepatitis C virus inhibitor, using feasible TLC-spectro-densitometry: application to pharmaceutical dosage form and human plasma
- PMID: 35518774
- PMCID: PMC9054297
- DOI: 10.1039/d0ra01172j
Stress stability study of simeprevir, a hepatitis C virus inhibitor, using feasible TLC-spectro-densitometry: application to pharmaceutical dosage form and human plasma
Abstract
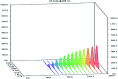
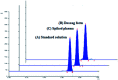
Simeprevir is one of the newest direct action anti-hepatitis C drugs. In the present work, a simple, highly selective and stability-indicating, high-performance thin-layer chromatography (HPTLC) method is proposed and validated for the assay of simeprevir both in pharmaceutical dosage form and spiked human plasma. The method used silica gel 60 F254 coated HPTLC aluminum plates as the stationary phase. The mobile phase system was ethyl acetate-hexane-methanol (5 : 4 : 1, v/v/v). The wavelength for both detection and quantitation was set at 288 nm. This system was found to give a compact spot of simeprevir; the retardation factor (R F) value was 0.67 ± 0.02. The guidelines of the International Conference on Harmonization were followed to validate the proposed analytical method, and the results were acceptable. The calibration curve was linear over the range of 80-1000 ng per spot. The limit of detection was 19.0 ng per spot, and the limit of quantitation was 57.0 ng per spot. The drug was subjected to various stress conditions including hydrolytic, oxidative and UV-induced resulting in varying degrees of degradation. The results showed that the proposed method could efficiently separate the degradation products from the intact drug and allow its satisfactory quantitation. The proposed method was employed successfully for the accurate and reproducible analysis of the pharmaceutical preparation and human plasma containing the drug. The proposed method's precision and accuracy were statistically similar to those of a reported method.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Feasible TLC-Spectro-Densitometry Technique for Simultaneous Determination of Two Hepatitis C Antiviral Drugs, Sofosbuvir and Simeprevir: Application to Combined Pharmaceutical Dosage Forms and Human Plasma.J Chromatogr Sci. 2021 May 20;59(6):576-583. doi: 10.1093/chromsci/bmab034. J Chromatogr Sci. 2021. PMID: 33822903
-
Application of a validated stability-indicating densitometric thin-layer chromatographic method to stress degradation studies on moxifloxacin.Anal Chim Acta. 2007 Jan 16;582(1):75-82. doi: 10.1016/j.aca.2006.08.053. Epub 2006 Sep 1. Anal Chim Acta. 2007. PMID: 17386477
-
Stability-Indicating TLC-densitometric determination of nebivolol hydrochloride in bulk and pharmaceutical dosage form.J Chromatogr Sci. 2010 Feb;48(2):109-13. doi: 10.1093/chromsci/48.2.109. J Chromatogr Sci. 2010. PMID: 20109287
-
Validated HPTLC method of analysis for artemether and its formulations.J Pharm Biomed Anal. 2007 Feb 19;43(3):839-44. doi: 10.1016/j.jpba.2006.08.029. Epub 2006 Oct 12. J Pharm Biomed Anal. 2007. PMID: 17045768
-
Application of stability-indicating HPTLC method for quantitative determination of metadoxine in pharmaceutical dosage form.Farmaco. 2005 Apr;60(4):351-60. doi: 10.1016/j.farmac.2005.01.001. Farmaco. 2005. PMID: 15848212
Cited by
-
Application of different spectrophotometric methods for quantitative analysis of direct acting antiviral drugs simeprevir and sofosbuvir.Spectrochim Acta A Mol Biomol Spectrosc. 2022 May 5;272:121012. doi: 10.1016/j.saa.2022.121012. Epub 2022 Feb 7. Spectrochim Acta A Mol Biomol Spectrosc. 2022. PMID: 35158141 Free PMC article.
-
Identification, isolation, structural characterization, in silico toxicity prediction and in vitro cytotoxicity assay of simeprevir acidic and oxidative degradation products.RSC Adv. 2020 Nov 24;10(70):42816-42826. doi: 10.1039/d0ra09253c. eCollection 2020 Nov 23. RSC Adv. 2020. PMID: 35514884 Free PMC article.
References
-
- PubChem Database, Simeprevir, 2019
LinkOut - more resources
Full Text Sources

