Chemical constituents from the stems of Machilus philippinensis Merr. and the neuroprotective activity of cinnamophilin
- PMID: 35518857
- PMCID: PMC9066449
- DOI: 10.1039/c9ra03514a
Chemical constituents from the stems of Machilus philippinensis Merr. and the neuroprotective activity of cinnamophilin
Abstract
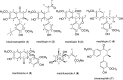
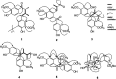
The Machilus genus (Lauraceae) had been extensively utilized in folk medicine due to its broad range of bioactivities. In the present study, a series of chromatographic separations of the methanol extract of stems of M. philippinensis led to the identification of thirty eight compounds totally. Among these, biscinnamophilin (1), machilupins A-C (2-4), machilutone A (5), and machilusoxide A (6) were new compounds reported for the first time. In addition, 5 was characterized with a unprecedented carbon skeleton. Other known compounds, including the major compounds cinnamophilin (7) and meso-dihydroguaiaretic acid (8), are identified by comparison of their physical and spectroscopic data with reported values. One of the reported compounds, cinnamophilin A (10), should be revised as dehydroguaiaretic acid (9) after careful comparison of all the 1H and 13C NMR data. Moreover, the neuroprotective activity of cinnamophilin (7) was examined in a primary cortical neuron culture and the results indicated that 7 was effective against glutamate induced excitotoxicity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Suppression of the SOS-Inducing Activity of Trp-P-1 and Aflatoxin B1 by Meso-dihydroguaiaretic Acid from Machilus thunbergii in the Salmonella typhimurium TA1535/pSK1002 umu Test.Biosci Biotechnol Biochem. 1998;62(7):1425-7. doi: 10.1271/bbb.62.1425. Biosci Biotechnol Biochem. 1998. PMID: 27397003
-
Neuroprotective lignans from the bark of Machilus thunbergii.Planta Med. 2004 Jan;70(1):79-80. doi: 10.1055/s-2004-815463. Planta Med. 2004. PMID: 14765301
-
Meso-dihydroguaiaretic acid from Machilus thunbergii SIEB et ZUCC., and its effects on the expression of matrix metalloproteinase-2, 9 cause by ultraviolet irradiated cultured human keratinocyte cells (HaCaT).Biol Pharm Bull. 2005 Nov;28(11):2176-9. doi: 10.1248/bpb.28.2176. Biol Pharm Bull. 2005. PMID: 16272716
-
Chemical constituents of plants from the genus Machilus.Chem Biodivers. 2011 Nov;8(11):1943-57. doi: 10.1002/cbdv.201000267. Chem Biodivers. 2011. PMID: 22083908 Review. No abstract available.
-
Mallotus philippinensis Muell. Arg (Euphorbiaceae): ethnopharmacology and phytochemistry review.Biomed Res Int. 2014;2014:213973. doi: 10.1155/2014/213973. Epub 2014 Jul 8. Biomed Res Int. 2014. PMID: 25105119 Free PMC article. Review.
Cited by
-
Cytotoxic clerodane diterpenoids from the roots of Casearia barteri Mast.RSC Adv. 2024 Jul 22;14(32):23109-23117. doi: 10.1039/d4ra04393f. eCollection 2024 Jul 19. RSC Adv. 2024. PMID: 39040697 Free PMC article.
References
LinkOut - more resources
Full Text Sources

