Efficient removal of crystal violet dye using EDTA/graphene oxide functionalized corncob: a novel low cost adsorbent
- PMID: 35518863
- PMCID: PMC9066744
- DOI: 10.1039/c9ra04003j
Efficient removal of crystal violet dye using EDTA/graphene oxide functionalized corncob: a novel low cost adsorbent
Abstract
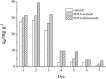
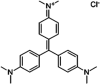
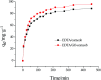
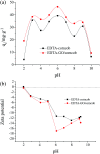
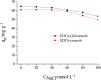
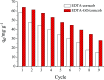
In this study, EDTA functionalized corncob (EDTA-corncob) and EDTA/graphene oxide functionalized corncob (EDTA-GO/corncob) were prepared using disodium ethylenediamine tetraacetic acid and the graphene oxide immersion method. EDTA-corncob and EDTA-GO/corncob were characterized by SEM and FTIR spectroscopy. On this basis, the adsorption properties of EDTA-corncob and EDTA-GO/corncob were studied with crystal violet as the adsorbate. The optimum adsorption conditions were determined by the effect of samples on the adsorption properties of crystal violet at different times, temperatures and pH, and the reusability of the samples was studied. The results showed that adsorption capacity of crystal violet on EDTA-GO/corncob was higher compared with natural corncob and EDTA-corncob. The most suitable pH value of the solution is about 6.0, the adsorption equilibrium time is 200 min. EDTA-GO/corncob can be reused eight times. This study indicated that EDTA-GO/corncob is a reusable adsorbent for rapid, low-cost, and efficient removal of dye from waste water.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Jafari A. J. Kakavandi B. Kalantary R. R. Gharibi H. Asadi A. Azari A. Babaei A. A. Takdastan A. Korean J. Chem. Eng. 2016;33(10):2878–2890. doi: 10.1007/s11814-016-0155-x. - DOI
-
- Zhang H. Luan Q. Tang H. Huang F. Zheng M. Deng Q. Xiang X. Yang C. Shi J. Zheng C. Zhou Q. Cellulose . 2017;24(2):903–914. doi: 10.1007/s10570-016-1129-1. - DOI
LinkOut - more resources
Full Text Sources

