Recent advances in the application of nano-catalysts for Hiyama cross-coupling reactions
- PMID: 35518942
- PMCID: PMC9060269
- DOI: 10.1039/c8ra08112c
Recent advances in the application of nano-catalysts for Hiyama cross-coupling reactions
Abstract
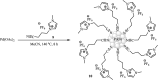
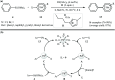
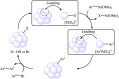
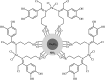
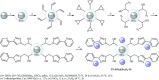
This mini-review highlights the recent developments in the field of metal nanoparticle (NP) catalyzed Hiyama cross-coupling reactions. Most of the nanocatalysts outlined here allow convenient and green synthetic pathways for the construction of carbon-carbon bonds in water and fluoride-free conditions. Literature has been surveyed from 2005 to February 2018.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

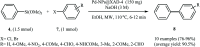

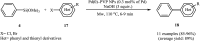
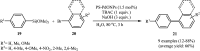
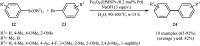

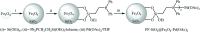
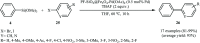
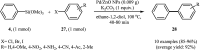

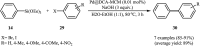


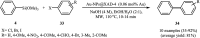
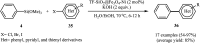
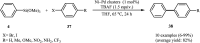







Similar articles
-
Transition Metal Catalyzed Hiyama Cross-Coupling: Recent Methodology Developments and Synthetic Applications.Molecules. 2022 Sep 2;27(17):5654. doi: 10.3390/molecules27175654. Molecules. 2022. PMID: 36080422 Free PMC article. Review.
-
Recent advances in noble metal nanocatalysts for Suzuki and Heck cross-coupling reactions.Molecules. 2010 Mar 25;15(4):2124-38. doi: 10.3390/molecules15042124. Molecules. 2010. PMID: 20428032 Free PMC article. Review.
-
A Review on Recent Advances in the Application of Nanocatalysts in A3 Coupling Reactions.Chem Rec. 2018 Oct;18(10):1409-1473. doi: 10.1002/tcr.201700100. Epub 2018 Mar 14. Chem Rec. 2018. PMID: 29537731 Review.
-
Recent progress in application of nanocatalysts for carbonylative Suzuki cross-coupling reactions.RSC Adv. 2021 Jan 21;11(4):2112-2125. doi: 10.1039/d0ra09846a. eCollection 2021 Jan 6. RSC Adv. 2021. PMID: 35424173 Free PMC article. Review.
-
Recent Progress in Application of Graphene Supported Metal Nanoparticles in C-C and C-X Coupling Reactions.Chem Rec. 2018 Feb;18(2):165-229. doi: 10.1002/tcr.201700022. Epub 2017 Jul 26. Chem Rec. 2018. PMID: 28745452 Review.
Cited by
-
Methods for direct C(sp2)-H bonds azidation.RSC Adv. 2019 Aug 13;9(43):25199-25215. doi: 10.1039/c9ra04534a. eCollection 2019 Aug 8. RSC Adv. 2019. PMID: 35528700 Free PMC article. Review.
-
Recent advances in direct trifluoromethylation of olefinic C-H bonds.RSC Adv. 2019 Sep 3;9(47):27625-27639. doi: 10.1039/c9ra04170b. eCollection 2019 Aug 29. RSC Adv. 2019. PMID: 35529210 Free PMC article. Review.
-
A fruitful century for the scalable synthesis and reactions of biphenyl derivatives: applications and biological aspects.RSC Adv. 2023 Jun 16;13(27):18262-18305. doi: 10.1039/d3ra03531j. eCollection 2023 Jun 15. RSC Adv. 2023. PMID: 37333795 Free PMC article. Review.
-
A walk around the application of nanocatalysts for cross-dehydrogenative coupling of C-H bonds.RSC Adv. 2019 Dec 16;9(71):41684-41702. doi: 10.1039/c9ra08752d. eCollection 2019 Dec 13. RSC Adv. 2019. PMID: 35557874 Free PMC article. Review.
-
Intermolecular difunctionalization of alkenes: synthesis of β-hydroxy sulfides.RSC Adv. 2021 Apr 7;11(22):13138-13151. doi: 10.1039/d0ra09848e. eCollection 2021 Apr 7. RSC Adv. 2021. PMID: 35423843 Free PMC article. Review.
References
-
- Diedrich F. and De Meijere A., Metal catalyzed cross-coupling reactions, vol. 1, Wiley-VCH Verlag GmbH, Weinheim, 2004
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

