DMSO-mediated palladium-catalyzed cyclization of two isothiocyanates via C-H sulfurization: a new route to 2-aminobenzothiazoles
- PMID: 35518944
- PMCID: PMC9060301
- DOI: 10.1039/c8ra09784d
DMSO-mediated palladium-catalyzed cyclization of two isothiocyanates via C-H sulfurization: a new route to 2-aminobenzothiazoles
Abstract
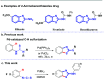
DMSO was found to activate arylisothiocyanates for self-nucleophilic addition. A subsequent intramolecular C-H sulfurization catalyzed by PdBr2 enables access to a wide range of 2-aminobenzothiazole derivatives in moderate to good yields. This is the first example of a DMSO-mediated Pd-catalyzed synthesis of 2-aminobenzothiazoles through cyclization/C-H sulfurization of two isothiocyanates.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Sharma P. C. Bansal K. K. Deep A. Pathak M. Curr. Top. Med. Chem. 2017;17:208–237. doi: 10.2174/1568026616666160530152546. - DOI - PubMed
- Sweeney J. B. Rattray M. Pugh V. Powell L. A. ACS Med. Chem. Lett. 2018;9:552–556. doi: 10.1021/acsmedchemlett.8b00103. - DOI - PMC - PubMed
- Hroch L. Guest P. Benek O. Soukup O. Janockova J. Dolezal R. Kuca K. Aitken L. Smith T. K. Gunn-Moore F. Zala D. Ramsay R. R. Musilek K. Bioorg. Med. Chem. 2017;25:1143–1152. doi: 10.1016/j.bmc.2016.12.029. - DOI - PubMed
- Ji Z. Zhou F. Wei S. Bioorg. Med. Chem. Lett. 2015;25:4065–4068. doi: 10.1016/j.bmcl.2015.08.051. - DOI - PubMed
-
-
Synthesis of benzothiazoles via C–H sulfurization, see:
- Jordan A. D. Luo C. Reitz A. B. J. Org. Chem. 2003;68:8693–8696. doi: 10.1021/jo0349431. - DOI - PubMed
- Joyce L. L. Batey R. A. Org. Lett. 2009;11:2792–2795. doi: 10.1021/ol900958z. - DOI - PubMed
- Inamoto K. Hasegawa C. Kawasaki J. Hiroya K. Doi T. Adv. Synth. Catal. 2010;352:2643–2655. doi: 10.1002/adsc.201000604. - DOI
- Wang H. Wang L. Shang J. Li X. Wang H. Gui J. Lei A. Chem. Commun. 2012;48:76–78. doi: 10.1039/C1CC16184A. - DOI - PubMed
- Sahoo S. K. Banerjee A. Chakraborty S. Patel B. K. ACS Catal. 2012;2:544–551. doi: 10.1021/cs200590p. - DOI
- Wang J. Zong Y. Zhang X. Gao Y. Li Z. Yue G. Quan Z. Wang X. Synlett. 2014;25:2143–2148. doi: 10.1055/s-0034-1378547. - DOI
- Sharma S. Pathare R. S. Maurya A. K. Gopal K. Roy T. K. Sawant D. M. Pardasani R. T. Org. Lett. 2016;18:356–359. doi: 10.1021/acs.orglett.5b03185. - DOI - PubMed
-
-
-
Synthesis of benzothiazoles via cross-coupling reaction of ortho-halothiourea, see:
- Benedi C. Bravo F. Uriz P. Fernández E. Claver C. Castillón S. Tetrahedron Lett. 2003;44:6073–6077. doi: 10.1016/S0040-4039(03)01469-2. - DOI
- Joyce L. L. Evindar G. Batey R. A. Chem. Commun. 2004:446–447. doi: 10.1039/B311591G. - DOI - PubMed
- Wang R. Yang W.-J. Yue L. Pan W. Zeng H.-Y. Synlett. 2012;23:1643–1648. doi: 10.1055/s-0031-1291159. - DOI
- Rout S. K. Guin S. Nath J. Patel B. K. Green Chem. 2012;14:2491–2498. doi: 10.1039/C2GC35575B. - DOI
- Min H. Xiao G. Liu W. Liang Y. Org. Biomol. Chem. 2016;14:11088–11091. doi: 10.1039/C6OB02413K. - DOI - PubMed
- Zeng W. Dang P. Zhang X. Liang Y. Peng C. RSC Adv. 2014;4:31003–31006. doi: 10.1039/C4RA04047C. - DOI
-
-
-
Metal-catalyzed tandem reaction of isothiocyanates with amines, see:
- Ding Q. He X. Wu J. J. Comb. Chem. 2009;11:587–591. doi: 10.1021/cc900027c. - DOI - PubMed
- Guo Y.-J. Tang R.-Y. Zhong P. Li J.-H. Tetrahedron Lett. 2010;51:649–652. doi: 10.1016/j.tetlet.2009.11.086. - DOI
- Khatun N. Jamir L. Ganesh M. Patel B. K. RSC Adv. 2012;2:11557–11565. doi: 10.1039/C2RA21826G. - DOI
- Chen L. Huang B. Nie Q. Cai M. Appl. Organomet. Chem. 2016;30:446–450. doi: 10.1002/aoc.3453. - DOI
- Zhao N. Liu L. Wang F. Li J. Zhang W. Adv. Synth. Catal. 2014;356:2575–2579. doi: 10.1002/adsc.201400043. - DOI
- Ding Q. Cao B. Liu X. Zong Z. Peng Y.-Y. Green Chem. 2010;12:1607–1610. doi: 10.1039/C0GC00123F. - DOI
-
LinkOut - more resources
Full Text Sources