Scope of organometallic compounds based on transition metal-arene systems as anticancer agents: starting from the classical paradigm to targeting multiple strategies
- PMID: 35518979
- PMCID: PMC9060267
- DOI: 10.1039/c8ra07926a
Scope of organometallic compounds based on transition metal-arene systems as anticancer agents: starting from the classical paradigm to targeting multiple strategies
Abstract
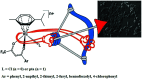
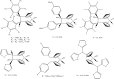
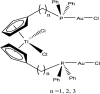
The advent of the clinically approved drug cisplatin started a new era in the design of metallodrugs for cancer chemotherapy. However, to date, there has not been much success in this field due to the persistence of some side effects and multi-drug resistance of cancer cells. In recent years, there has been increasing interest in the design of metal chemotherapeutics using organometallic complexes due to their good stability and unique properties in comparison to normal coordination complexes. Their intermediate properties between that of traditional inorganic and organic materials provide researchers with a new platform for the development of more promising cancer therapeutics. Classical metal-based drugs exert their therapeutic potential by targeting only DNA, but in the case of organometallic complexes, their molecular target is quite distinct to avoid drug resistance by cancer cells. Some organometallic drugs act by targeting a protein or inhibition of enzymes such as thioredoxin reductase (TrRx), while some target mitochondria and endoplasmic reticulum. In this review, we mainly discuss organometallic complexes of Ru, Ti, Au, Fe and Os and their mechanisms of action and how new approaches improve their therapeutic potential towards various cancer phenotypes. Herein, we discuss the role of structure-reactivity relationships in enhancing the anticancer potential of drugs for the benefit of humans both in vitro and in vivo. Besides, we also include in vivo tumor models that mimic human physiology to accelerate the development of more efficient clinical organometallic chemotherapeutics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
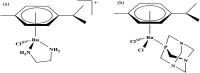
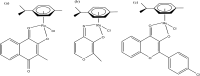
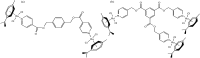
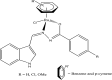
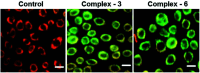




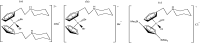



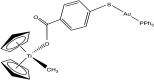
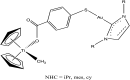
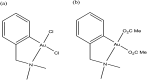


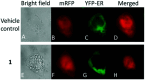
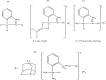
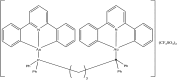

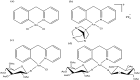
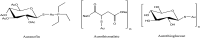
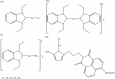
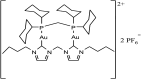

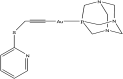


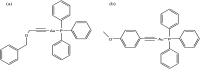



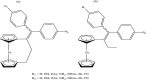
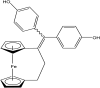

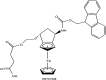
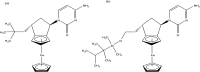
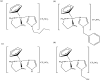
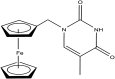
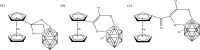
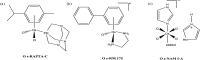
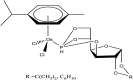
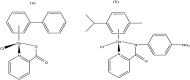
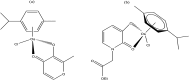
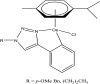
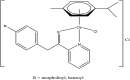

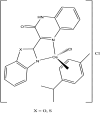
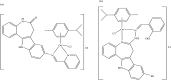



References
-
- Jaouen G., Bioorganometallics, Wiley-VCH, Weinheim (Germany), 2006
-
- Jaouen G. and Dyson P., in Comprehensive Organometallic Chemistry III, ed. R. H. Crabtree and D. M. P. Mingos, Elsevier Ltd., Oxford, 2007, vol. 12, p. 445
-
- Hartinger C. G. Metzler-Nolte N. Dyson P. J. Organometallics. 2012;31:5677–5685. doi: 10.1021/om300373t. - DOI
-
- Allardyce C. S. Dorcier A. Scolaro C. Dyson P. J. Appl. Organomet. Chem. 2005;19:1–10. doi: 10.1002/aoc.725. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

