β-Myrcene/isobornyl methacrylate SG1 nitroxide-mediated controlled radical polymerization: synthesis and characterization of gradient, diblock and triblock copolymers
- PMID: 35518984
- PMCID: PMC9060242
- DOI: 10.1039/c8ra09192g
β-Myrcene/isobornyl methacrylate SG1 nitroxide-mediated controlled radical polymerization: synthesis and characterization of gradient, diblock and triblock copolymers
Abstract
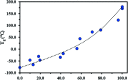
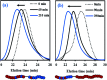
β-Myrcene (My), a natural 1,3-diene, and isobornyl methacrylate (IBOMA), from partially bio-based raw materials sources, were copolymerized by nitroxide-mediated polymerization (NMP) in bulk using the SG1-based BlocBuilder™ alkoxyamine functionalized with an N-succinimidyl ester group, NHS-BlocBuilder, at T = 100 °C with initial IBOMA molar feed compositions f IBOMA,0 = 0.10-0.90. Copolymer reactivity ratios were r My = 1.90-2.16 and r IBOMA = 0.02-0.07 using Fineman-Ross, Kelen-Tudos and non-linear least-squares fitting to the Mayo-Lewis terminal model and indicated the possibility of gradient My/IBOMA copolymers. A linear increase in molecular weight versus conversion and a low dispersity (Đ ≤ 1.41) were exhibited by My/IBOMA copolymerization with f IBOMA,0 ≤ 0.80. My-rich and IBOMA-rich copolymers were shown to have a high degree of chain-end fidelity by performing subsequent chain-extensions with IBOMA and/or My, and by 31P NMR analysis. The preparation by NMP of My/IBOMA thermoplastic elastomers (TPEs), mostly bio-sourced, was then attempted. IBOMA-My-IBOMA triblock copolymers containing a minor fraction of My or styrene (S) units in the outer hard segments (M n = 51-95 kg mol-1, Đ = 1.91-2.23 and F IBOMA = 0.28-0.36) were synthesized using SG1-terminated poly(ethylene-stat-butylene) dialkoxyamine. The micro-phase separation was suggested by the detection of two distinct T gs at about -60 °C and +180 °C and confirmed by atomic force microscopy (AFM). A plastic stress-strain behavior (stress at break σ B = 3.90 ± 0.22 MPa, elongation at break ε B = 490 ± 31%) associated to an upper service temperature of about 140 °C were also highlighted for these triblock polymers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Szwarc M. Nature. 1956;178:1168–1169. doi: 10.1038/1781168a0. - DOI
-
- Szwarc M. Levy M. Milkovich R. J. Am. Chem. Soc. 1956;78:2656–2657. doi: 10.1021/ja01592a101. - DOI
-
- Quirk R. P., Zhuo Q., Jang S. H., Lee Y. and Lizarraga G., in Applications of Anionic Polymerization Research, American Chemical Society, 1998, ch. 1, pp. 2–27
-
- Elias H.-G. in Macromolecules: Synthesis, Materials, and Technology, Springer, 2nd edn, 1984, ch. 20, pp. 681–736
-
- Chiefari J. Chong Y. Ercole F. Krstina J. Jeffery J. Le T. Mayadunne R. Meijs G. Moad C. Moad G. Rizzardo E. Thang S. Macromolecules. 1998;31:5559–5562. doi: 10.1021/ma9804951. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

