Recent advances and prospects in the palladium-catalyzed cyanation of aryl halides
- PMID: 35519018
- PMCID: PMC9056778
- DOI: 10.1039/d0ra05960a
Recent advances and prospects in the palladium-catalyzed cyanation of aryl halides
Abstract
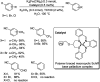
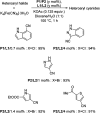
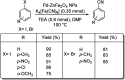
Aryl nitriles are compounds with wide significance. They have made their own space in various sectors including pharmaceuticals, industries, natural product chemistry, and so on. Furthermore, they are also key intermediates in various transformations in organic chemistry. Transition metal-catalyzed cyanation reactions have proved to be a better replacement for the existing traditional synthetic strategies for aryl nitriles. Palladium is one of the most studied transition metals other than copper and nickel owing to its wide functional group compatibility and catalytic efficacy. There have been drastic developments in the field of palladium-catalyzed cyanation since its discovery in the 1973. This review summarizes the recent developments in the palladium-catalyzed cyanation of aryl halides and covers literature from 2012-2020.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

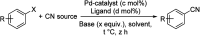






























Similar articles
-
Nickel-Catalyzed Cyanation of Aryl Halides.Molecules. 2025 Aug 20;30(16):3440. doi: 10.3390/molecules30163440. Molecules. 2025. PMID: 40871592 Free PMC article. Review.
-
Recent developments and perspectives in palladium-catalyzed cyanation of aryl halides: synthesis of benzonitriles.Chem Soc Rev. 2011 Oct;40(10):5049-67. doi: 10.1039/c1cs15004a. Epub 2011 Apr 28. Chem Soc Rev. 2011. PMID: 21528150 Review.
-
Decarbonylative Cyanation of Amides by Palladium Catalysis.Org Lett. 2017 Jun 16;19(12):3095-3098. doi: 10.1021/acs.orglett.7b01199. Epub 2017 Jun 1. Org Lett. 2017. PMID: 28569059
-
Nickel-Catalyzed Reductive Cyanation of Aryl Halides and Epoxides with Cyanogen Bromide.Molecules. 2024 Dec 20;29(24):6016. doi: 10.3390/molecules29246016. Molecules. 2024. PMID: 39770100 Free PMC article.
-
Mild palladium-catalyzed cyanation of (hetero)aryl halides and triflates in aqueous media.Org Lett. 2015 Jan 16;17(2):202-5. doi: 10.1021/ol5032359. Epub 2015 Jan 2. Org Lett. 2015. PMID: 25555140 Free PMC article.
Cited by
-
Ni-Catalyzed Cyanation of (Hetero)Aryl Electrophiles Using the Nontoxic Cyanating Reagent K4[Fe(CN)6].ACS Catal. 2025 Apr 5;15(8):6459-6465. doi: 10.1021/acscatal.5c00158. eCollection 2025 Apr 18. ACS Catal. 2025. PMID: 40270882 Free PMC article.
-
Open-Source Chromatographic Data Analysis for Reaction Optimization and Screening.ACS Cent Sci. 2023 Feb 9;9(2):307-317. doi: 10.1021/acscentsci.2c01042. eCollection 2023 Feb 22. ACS Cent Sci. 2023. PMID: 36844498 Free PMC article.
-
Visible-Light-Promoted Direct C3-H Cyanomethylation of 2H-Indazoles.ACS Omega. 2023 Mar 13;8(12):11192-11200. doi: 10.1021/acsomega.2c08094. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008106 Free PMC article.
-
Catalytic atroposelective synthesis of axially chiral benzonitriles via chirality control during bond dissociation and CN group formation.Nat Commun. 2022 Jan 10;13(1):36. doi: 10.1038/s41467-021-27813-4. Nat Commun. 2022. PMID: 35013312 Free PMC article.
-
Synthesis and characterization of a green and recyclable arginine-based palladium/CoFe2O4 nanomagnetic catalyst for efficient cyanation of aryl halides.RSC Adv. 2024 May 10;14(20):14139-14151. doi: 10.1039/d4ra01200c. eCollection 2024 Apr 25. RSC Adv. 2024. PMID: 38737408 Free PMC article.
References
-
- Murahashi S. I., Science of Synthesis, Georg Thieme, Stuttgart, 2004, vol. 19, p. 345
- Kleemann A., Engel J., Kutscher B. and Reichert D., Pharmaceutical Substances: Syntheses, Patents, Applications, Georg Thieme Verlag, Stuttgart, 4th edn, 2001, p. 241
-
- Industrial Biotransformations, ed. A. Liese, K. Seelbach and C. Wandrey, Wiley-VCH, Weinheim, Germany, 2nd edn, 2006
-
- Fatiadi J., in Preparation and Synthetic Applications of Cyano Compounds, ed. S. Patai and Z. Rappoport, Wiley-VCH, New York, NY, 1983
- Rappoport Z., in Chemistry of the Cyano Group, John Wiley & Sons, London, UK, 1970, p. 121
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

