DFT study of α-Keggin, lacunary Keggin, and ironII-VI substituted Keggin polyoxometalates: the effect of oxidation state and axial ligand on geometry, electronic structures and oxygen transfer
- PMID: 35519024
- PMCID: PMC9056712
- DOI: 10.1039/d0ra05189f
DFT study of α-Keggin, lacunary Keggin, and ironII-VI substituted Keggin polyoxometalates: the effect of oxidation state and axial ligand on geometry, electronic structures and oxygen transfer
Abstract
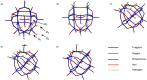
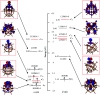
Herein, the geometry, electronic structure, Fe-ligand bonding nature and simulated IR spectrum of α-Keggin, lacunary Keggin, iron(ii/iii)-substituted and the important oxidized high-valent iron derivatives of Keggin type polyoxometalates have been studied using the density functional theory (DFT/OPTX-PBE) method and natural bond orbital (NBO) analysis. The effects of different Fe oxidation states (ii-vi) and H2O/OH-/O2- ligand interactions have been addressed concerning their geometry and electronic structures. It has been revealed that the d-atomic orbitals of Fe and 2p orbitals of polyoxometalate's oxygen-atoms contribute in ligand binding. Compared with other high valent species, the considered polyoxometalate system of [PW11O39(FeVO)]4-, possesses a high reactivity for oxygen transfer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Quantum chemical studies on high-valent metal nitrido derivatives of Keggin-type polyoxometalates ([PW11O39{M(VI)N}]4- (M = Ru, Os, Re)): M(VI)-N bonding and electronic structures.Inorg Chem. 2009 Jan 19;48(2):541-8. doi: 10.1021/ic8012443. Inorg Chem. 2009. PMID: 19072297
-
Theoretical studies on nitrido ruthenium (VI) porphyrin and high valent ruthenium nitrido derivatives of Keggin typical polyoxometalate ([PW11O39{Ru(VI)N}]4-): electronic structures and bonding features.Dalton Trans. 2009 Aug 21;(31):6208-13. doi: 10.1039/b902837d. Epub 2009 Jun 24. Dalton Trans. 2009. PMID: 20449118
-
Quantum chemical characterization of the generation of high-valent oxoruthenium species of Keggin type polyoxometalates: electronic structure and bonding features.Dalton Trans. 2011 Mar 28;40(12):2967-74. doi: 10.1039/c0dt01085e. Epub 2011 Feb 15. Dalton Trans. 2011. PMID: 21327240
-
Roles of Organic Fragments in Redirecting Crystal/Molecular Structures of Inorganic-Organic Hybrids Based on Lacunary Keggin-Type Polyoxometalates.Molecules. 2021 Oct 2;26(19):5994. doi: 10.3390/molecules26195994. Molecules. 2021. PMID: 34641537 Free PMC article. Review.
-
The cyclic 48-tungsto-8-phosphate [H7P8W48O184]33- Contant-Tézé polyanion and its derivatives [H6P4W24O94]18- and [H2P2W12O48]12-: structural aspects and reactivity.Dalton Trans. 2025 Mar 25;54(13):5208-5233. doi: 10.1039/d4dt03448a. Dalton Trans. 2025. PMID: 39936289 Review.
Cited by
-
Photophysical properties of Pt(ii) complexes based on the benzoquinoline (bzq) ligand with OLED implication: a theoretical study.RSC Adv. 2024 Jun 25;14(28):20278-20289. doi: 10.1039/d4ra03334e. eCollection 2024 Jun 18. RSC Adv. 2024. PMID: 38919282 Free PMC article.
References
LinkOut - more resources
Full Text Sources

