DFT study of α-Keggin, lacunary Keggin, and ironII-VI substituted Keggin polyoxometalates: the effect of oxidation state and axial ligand on geometry, electronic structures and oxygen transfer
- PMID: 35519024
- PMCID: PMC9056712
- DOI: 10.1039/d0ra05189f
DFT study of α-Keggin, lacunary Keggin, and ironII-VI substituted Keggin polyoxometalates: the effect of oxidation state and axial ligand on geometry, electronic structures and oxygen transfer
Abstract
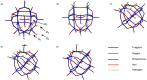
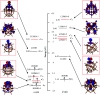
Herein, the geometry, electronic structure, Fe-ligand bonding nature and simulated IR spectrum of α-Keggin, lacunary Keggin, iron(ii/iii)-substituted and the important oxidized high-valent iron derivatives of Keggin type polyoxometalates have been studied using the density functional theory (DFT/OPTX-PBE) method and natural bond orbital (NBO) analysis. The effects of different Fe oxidation states (ii-vi) and H2O/OH-/O2- ligand interactions have been addressed concerning their geometry and electronic structures. It has been revealed that the d-atomic orbitals of Fe and 2p orbitals of polyoxometalate's oxygen-atoms contribute in ligand binding. Compared with other high valent species, the considered polyoxometalate system of [PW11O39(FeVO)]4-, possesses a high reactivity for oxygen transfer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources

