Synthesis, biological evaluation, and in silico studies of novel chalcone- and pyrazoline-based 1,3,5-triazines as potential anticancer agents
- PMID: 35519030
- PMCID: PMC9056798
- DOI: 10.1039/d0ra06799g
Synthesis, biological evaluation, and in silico studies of novel chalcone- and pyrazoline-based 1,3,5-triazines as potential anticancer agents
Abstract
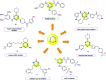
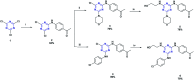
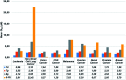
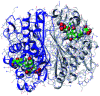
A novel series of triazin-chalcones (7,8)a-g and triazin-N-(3,5-dichlorophenyl)pyrazolines (9,10)a-g were synthesized and evaluated for their anticancer activity against nine different cancer strains. Triazine ketones 5 and 6 were synthesized from the cyanuric chloride 1 by using stepwise nucleophilic substitution of the chlorine atom. These ketones were subsequently subjected to a Claisen-Schmidt condensation reaction with aromatic aldehydes affording chalcones (7,8)a-g. Then, N-(3,5-dichlorophenyl)pyrazolines (9,10)a-g were obtained by cyclocondensation reactions of the respective chalcones (7,8)a-g with 3,5-dichlorophenylhydrazine. Among all the evaluated compounds, chalcones 7d,g and 8g exhibited more potent in vitro anticancer activity, with outstanding GI50 values ranging from 0.422 to 14.9 μM and LC50 values ranging from 5.08 μM to >100 μM. In silico studies, for both ligand- and structure-based, were executed to explore the inhibitory nature of chalcones and triazine derivatives. The results suggested that the evaluated compounds could act as modulators of the human thymidylate synthase enzyme.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Synthesis of Novel Triazine-Based Chalcones and 8,9-dihydro-7H-pyrimido[4,5-b][1,4]diazepines as Potential Leads in the Search of Anticancer, Antibacterial and Antifungal Agents.Int J Mol Sci. 2024 Mar 23;25(7):3623. doi: 10.3390/ijms25073623. Int J Mol Sci. 2024. PMID: 38612435 Free PMC article.
-
Synthesis of New 1,3,5-Triazine-Based 2-Pyrazolines as Potential Anticancer Agents.Molecules. 2018 Aug 6;23(8):1956. doi: 10.3390/molecules23081956. Molecules. 2018. PMID: 30082588 Free PMC article.
-
Synthesis, Anticancer and Antitubercular Properties of New Chalcones and Their Nitrogen-Containing Five-Membered Heterocyclic Hybrids Bearing Sulfonamide Moiety.Int J Mol Sci. 2022 Oct 20;23(20):12589. doi: 10.3390/ijms232012589. Int J Mol Sci. 2022. PMID: 36293443 Free PMC article.
-
Synthesis and biological evaluation of chalcone derivatives (mini review).Mini Rev Med Chem. 2012 Nov;12(13):1394-403. doi: 10.2174/13895575112091394. Mini Rev Med Chem. 2012. PMID: 22876958 Review.
-
Recent Updates on Pyrazoline Derivatives as Promising Candidates for Neuropsychiatric and Neurodegenerative Disorders.Curr Top Med Chem. 2021;21(30):2695-2714. doi: 10.2174/1568026621999210902123132. Curr Top Med Chem. 2021. PMID: 34477522 Review.
Cited by
-
Synthesis of Novel Triazine-Based Chalcones and 8,9-dihydro-7H-pyrimido[4,5-b][1,4]diazepines as Potential Leads in the Search of Anticancer, Antibacterial and Antifungal Agents.Int J Mol Sci. 2024 Mar 23;25(7):3623. doi: 10.3390/ijms25073623. Int J Mol Sci. 2024. PMID: 38612435 Free PMC article.
-
Five and six membered heterocyclic rings endowed with azobenzene as dual EGFRT790M and VEGFR-2 inhibitors: design, synthesis, in silico ADMET profile, molecular docking, dynamic simulation and anticancer evaluations.RSC Adv. 2023 Dec 4;13(50):35321-35338. doi: 10.1039/d3ra06614b. eCollection 2023 Nov 30. RSC Adv. 2023. PMID: 38053688 Free PMC article.
-
1,3,5-Triazine: Recent Development in Synthesis of its Analogs and Biological Profile.Mini Rev Med Chem. 2024;24(22):2019-2071. doi: 10.2174/0113895575309800240526180356. Mini Rev Med Chem. 2024. PMID: 38847171 Review.
-
Recent Advances in the Biological Activity of s-Triazine Core Compounds.Pharmaceuticals (Basel). 2022 Feb 12;15(2):221. doi: 10.3390/ph15020221. Pharmaceuticals (Basel). 2022. PMID: 35215333 Free PMC article. Review.
-
New Benzimidazole-, 1,2,4-Triazole-, and 1,3,5-Triazine-Based Derivatives as Potential EGFRWT and EGFRT790M Inhibitors: Microwave-Assisted Synthesis, Anticancer Evaluation, and Molecular Docking Study.ACS Omega. 2022 Feb 18;7(8):7155-7171. doi: 10.1021/acsomega.1c06836. eCollection 2022 Mar 1. ACS Omega. 2022. PMID: 35252706 Free PMC article.
References
LinkOut - more resources
Full Text Sources

