Effect of localized UV irradiation on the crystallinity and electrical properties of dip-coated polythiophene thin films
- PMID: 35519062
- PMCID: PMC9056789
- DOI: 10.1039/d0ra06339h
Effect of localized UV irradiation on the crystallinity and electrical properties of dip-coated polythiophene thin films
Abstract
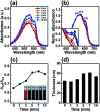
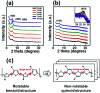
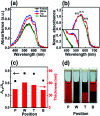
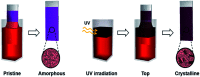
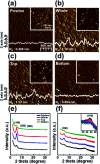
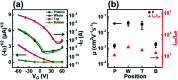
Ensuring high performance in polymer devices requires conjugated polymers with interchain π-π stacking interactions via van der Waals forces, which can induce structural changes in the polymer thin film. Here, we present a systematic study of using simple localized UV irradiation to overcome the low crystallinity and poor charge carrier transport in dip-coated poly(3-hexylthiophene) (P3HT) thin films, which are consequences of the limited selection of solvents compatible with the dip-coating process. UV irradiation for only a few minutes effectively promoted P3HT chain self-assembly and association in the solution state. Brief UV irradiation of a P3HT solution led to well-ordered molecular structures in the resultant P3HT films dip-coated using a low boiling point solvent with rapid solvent evaporation. In addition, the position at which UV light was irradiated on the dip-coating solutions was varied, and the effects of the irradiation position and time on the crystallinity and electrical properties of the resultant P3HT thin films were investigated.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Clarifying the Dominant Role of Crystallinity and Molecular Orientation in Differently Processed Thin Films of Regioregular Poly(3-hexylthiophene).Micromachines (Basel). 2024 May 22;15(6):677. doi: 10.3390/mi15060677. Micromachines (Basel). 2024. PMID: 38930647 Free PMC article.
-
Solvent Additive-Assisted Anisotropic Assembly and Enhanced Charge Transport of π-Conjugated Polymer Thin Films.ACS Appl Mater Interfaces. 2018 May 30;10(21):18131-18140. doi: 10.1021/acsami.8b03221. Epub 2018 May 16. ACS Appl Mater Interfaces. 2018. PMID: 29726258
-
Conjugated Block Copolymers for Functional Nanostructures.Acc Chem Res. 2022 Aug 16;55(16):2224-2234. doi: 10.1021/acs.accounts.2c00244. Epub 2022 Aug 3. Acc Chem Res. 2022. PMID: 35921179
-
Interfacial and confined molecular-assembly of poly(3-hexylthiophene) and its application in organic electronic devices.Sci Technol Adv Mater. 2022 Sep 27;23(1):619-632. doi: 10.1080/14686996.2022.2125826. eCollection 2022. Sci Technol Adv Mater. 2022. PMID: 36212681 Free PMC article. Review.
-
What Is the Assembly Pathway of a Conjugated Polymer From Solution to Thin Films?Front Chem. 2020 Dec 22;8:583521. doi: 10.3389/fchem.2020.583521. eCollection 2020. Front Chem. 2020. PMID: 33425847 Free PMC article. Review.
Cited by
-
Harnessing Compositional Gradients to Elucidate Phase Behaviors toward High Performance Polymer Semiconductor Blends.ACS Appl Electron Mater. 2024 Aug 13;6(8):5661-5671. doi: 10.1021/acsaelm.4c00680. eCollection 2024 Aug 27. ACS Appl Electron Mater. 2024. PMID: 39221137 Free PMC article.
References
-
- Holliday S. Donaghey J. E. McCulloch I. Chem. Mater. 2014;26:647–663. doi: 10.1021/cm402421p. - DOI
-
- Sirringhaus H. Adv. Mater. 2005;17:2411–2425. doi: 10.1002/adma.200501152. - DOI
-
- Jang J. Nam S. Im K. Hur J. Cha S. N. Kim J. Son H. B. Suh H. Loth M. A. Anthony J. E. Park J. J. Park C. E. Kim J. M. Kim K. Adv. Funct. Mater. 2012;22:1005–1014. doi: 10.1002/adfm.201102284. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

