A voltammetric sensor based on reduced graphene oxide-hemin-Ag nanocomposites for sensitive determination of tyrosine
- PMID: 35519092
- PMCID: PMC9055674
- DOI: 10.1039/d0ra04976j
A voltammetric sensor based on reduced graphene oxide-hemin-Ag nanocomposites for sensitive determination of tyrosine
Abstract
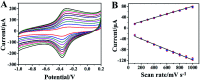
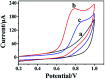
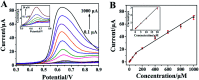
A novel voltammetric sensor was designed and used for the determination of l-tyrosine (l-Tyr) by surface modification of a glassy carbon electrode with reduced graphene oxide-hemin-Ag (rGO-H-Ag) nanocomposites. The nanocomposites were synthesized by a facile one-pot hydrothermal method and characterized by means of transmission electron microscopy and Raman spectroscopy. The determination of l-Tyr was investigated by cyclic voltammetry and further quantified using differential pulse voltammetry. The results revealed a significant enhanced electrochemical oxidation effect for l-Tyr at the nanocomposites modified electrode. Two linear ranges from 0.1 to 100 μM and 100 to 1000 μM as well as a low detection limit of 30 nM (S/N = 3) were obtained. In addition, the sensor also demonstrated good selectivity, reproducibility and stability.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
A glassy carbon electrode modified with a composite consisting of gold nanoparticle, reduced graphene oxide and poly(L-arginine) for simultaneous voltammetric determination of dopamine, serotonin and L-tryptophan.Mikrochim Acta. 2018 Aug 30;185(9):439. doi: 10.1007/s00604-018-2979-z. Mikrochim Acta. 2018. PMID: 30167981
-
Highly sensitive electrochemical aptasensor for Glypican-3 based on reduced graphene oxide-hemin nanocomposites modified on screen-printed electrode surface.Bioelectrochemistry. 2021 Apr;138:107696. doi: 10.1016/j.bioelechem.2020.107696. Epub 2020 Nov 17. Bioelectrochemistry. 2021. PMID: 33254049
-
Spherical Silver Nanoparticles Located on Reduced Graphene Oxide Nanocomposites as Sensitive Electrochemical Sensors for Detection of L-Cysteine.Sensors (Basel). 2024 Mar 10;24(6):1789. doi: 10.3390/s24061789. Sensors (Basel). 2024. PMID: 38544052 Free PMC article.
-
An electrochemical daunorubicin sensor based on the use of platinum nanoparticles loaded onto a nanocomposite prepared from nitrogen decorated reduced graphene oxide and single-walled carbon nanotubes.Mikrochim Acta. 2019 May 2;186(5):321. doi: 10.1007/s00604-019-3456-z. Mikrochim Acta. 2019. PMID: 31049702
-
Glassy carbon electrodes modified with reduced graphene oxide-MoS2-poly (3, 4-ethylene dioxythiophene) nanocomposites for the non-enzymatic detection of nitrite in water and milk.Anal Chim Acta. 2020 Jan 6;1093:93-105. doi: 10.1016/j.aca.2019.09.043. Epub 2019 Sep 19. Anal Chim Acta. 2020. PMID: 31735219
Cited by
-
An electrochemical platform based on a hemin-rGO-cMWCNTs modified aptasensor for sensitive detection of kanamycin.RSC Adv. 2021 Apr 28;11(26):15817-15824. doi: 10.1039/d1ra01135a. eCollection 2021 Apr 26. RSC Adv. 2021. PMID: 35481218 Free PMC article.
-
Manganese tetraphenylporphyrin and carbon nanocoil interface-based electrochemical sensing of tyrosine.RSC Adv. 2024 Aug 1;14(33):24105-24114. doi: 10.1039/d4ra02048k. eCollection 2024 Jul 26. RSC Adv. 2024. PMID: 39131187 Free PMC article.
-
Electrochemical Amino Acid Sensing: A Review on Challenges and Achievements.Biosensors (Basel). 2021 Dec 7;11(12):502. doi: 10.3390/bios11120502. Biosensors (Basel). 2021. PMID: 34940259 Free PMC article. Review.
-
Macromolecule-Nanoparticle-Based Hybrid Materials for Biosensor Applications.Biosensors (Basel). 2024 May 28;14(6):277. doi: 10.3390/bios14060277. Biosensors (Basel). 2024. PMID: 38920581 Free PMC article. Review.
-
Hemin as a Molecular Probe for Nitric Oxide Detection in Physiological Solutions: Experimental and Theoretical Assessment.Anal Chem. 2024 May 14;96(19):7763-7771. doi: 10.1021/acs.analchem.4c01516. Epub 2024 May 3. Anal Chem. 2024. PMID: 38699865 Free PMC article.
References
-
- Femstrom H. F. Femstrom J. D. Life Sci. 1995;57:97.
-
- Yokus Ö. A. Kardas F. Akyıldırım O. Eren T. Atar N. Yola M. L. Sens. Actuators, B. 2016;233:47. doi: 10.1016/j.snb.2016.04.050. - DOI
-
- Zhou S. Wu H. Wu Y. Shi H. Feng X. Huang H. Li J. Song W. Electrochim. Acta. 2013;112:90. doi: 10.1016/j.electacta.2013.08.134. - DOI
LinkOut - more resources
Full Text Sources

