The preparation of a difunctional porous β-tricalcium phosphate scaffold with excellent compressive strength and antibacterial properties
- PMID: 35519120
- PMCID: PMC9055648
- DOI: 10.1039/d0ra02388d
The preparation of a difunctional porous β-tricalcium phosphate scaffold with excellent compressive strength and antibacterial properties
Abstract
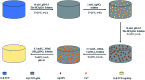
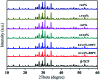
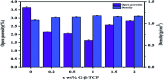
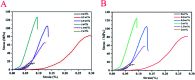
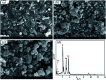
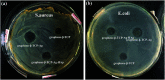
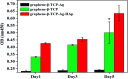
Porous β-tricalcium phosphate (β-Ca3(PO4)2, β-TCP) scaffolds are widely applied in the field of bone tissue engineering due to their nontoxicity, degradability, biocompatibility, and osteoinductivity. However, poor compressive strength and a lack of antibacterial properties have hindered their clinical application. In order to address these disadvantages, graphene (G) and silver nanoparticles were introduced into β-TCP through a two-step method. In the synthesis process, G-β-TCP was prepared via an in situ synthesis method, and then silver nanoparticles and HAp particles were coated on the surface of the G-β-TCP scaffold in an orderly fashion using dopamine as a binder. From the results of characterization, when the content of graphene was 1 wt% of β-TCP, the G-β-TCP scaffold had the highest compression strength (127.25 MPa). And core-shell G-β-TCP-Ag-HAp not only had reduced cytotoxicity via the continuous release of Ag+, but it also achieved long-term antibacterial properties. Besides, the material still showed good cell activity and proliferation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Beta-tricalcium phosphate enhanced mechanical and biological properties of 3D-printed polyhydroxyalkanoates scaffold for bone tissue engineering.Int J Biol Macromol. 2022 Jun 1;209(Pt A):1553-1561. doi: 10.1016/j.ijbiomac.2022.04.056. Epub 2022 Apr 18. Int J Biol Macromol. 2022. PMID: 35439474
-
Fabrication of Porous α-TCP/Gellan Gum Scaffold for Bone Tissue Engineering.J Nanosci Nanotechnol. 2016 Mar;16(3):3077-83. doi: 10.1166/jnn.2016.12463. J Nanosci Nanotechnol. 2016. PMID: 27455764
-
β-Tricalcium phosphate/poly(glycerol sebacate) scaffolds with robust mechanical property for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2015 Nov 1;56:37-47. doi: 10.1016/j.msec.2015.05.083. Epub 2015 Jun 11. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 26249563
-
In-vitroassessment of β-tricalcium phosphate/bredigite-ciprofloxacin (CPFX) scaffolds for bone treatment applications.Biomed Mater. 2021 Jun 14;16(4). doi: 10.1088/1748-605X/ac0590. Biomed Mater. 2021. PMID: 34038876
-
Synthesis of calcium phosphate-zirconia scaffold and human endometrial adult stem cells for bone tissue engineering.Artif Cells Nanomed Biotechnol. 2016;44(1):66-73. doi: 10.3109/21691401.2014.909825. Epub 2014 May 8. Artif Cells Nanomed Biotechnol. 2016. PMID: 24810360
Cited by
-
Preparation and characterization of permeability and mechanical properties of three-dimensional porous stainless steel.RSC Adv. 2022 Oct 3;12(43):28079-28087. doi: 10.1039/d2ra03893e. eCollection 2022 Sep 28. RSC Adv. 2022. PMID: 36320271 Free PMC article.
-
Reinforcing β-tricalcium phosphate scaffolds for potential applications in bone tissue engineering: impact of functionalized multi-walled carbon nanotubes.Sci Rep. 2024 Aug 17;14(1):19055. doi: 10.1038/s41598-024-68419-2. Sci Rep. 2024. PMID: 39154029 Free PMC article.
-
Antimicrobial and Cell-Friendly Properties of Cobalt and Nickel-Doped Tricalcium Phosphate Ceramics.Biomimetics (Basel). 2023 Dec 31;9(1):14. doi: 10.3390/biomimetics9010014. Biomimetics (Basel). 2023. PMID: 38248588 Free PMC article.
-
Clindamycin-Loaded Halloysite Nanotubes as the Antibacterial Component of Composite Hydrogel for Bone Repair.Polymers (Basel). 2022 Nov 26;14(23):5151. doi: 10.3390/polym14235151. Polymers (Basel). 2022. PMID: 36501546 Free PMC article.
References
LinkOut - more resources
Full Text Sources

