Selective photocatalytic oxidation of aromatic alcohols to aldehydes with air by magnetic WO3ZnO/Fe3O4. In situ photochemical synthesis of 2-substituted benzimidazoles
- PMID: 35519184
- PMCID: PMC9057692
- DOI: 10.1039/d0ra08403d
Selective photocatalytic oxidation of aromatic alcohols to aldehydes with air by magnetic WO3ZnO/Fe3O4. In situ photochemical synthesis of 2-substituted benzimidazoles
Abstract
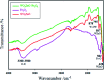
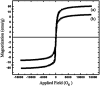
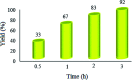
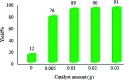
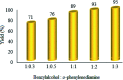
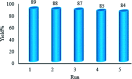
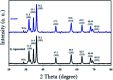
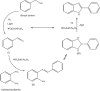
Recently, visible light-driven organic photochemical synthesis has been a pioneering field of interest from academic and industrial associations due to its unique features of green and sustainable chemistry. Herein, WO3ZnO/Fe3O4 was synthesized, characterized, and used as an efficient magnetic photocatalyst in the preparation of a range of 2-substituted benzimidazoles via the condensation of benzyl alcohol and o-phenylenediamine in ethanol at room temperature for the first time. The key feature of this work is focused on the in situ photocatalytic oxidation of benzyl alcohols to benzaldehydes under atmospheric air and in the absence of any further oxidant. This new heterogeneous nanophotocatalyst was characterized via XRD, FT-IR, VSM and SEM. Short reaction time, cost-effectiveness, broad substrate scope, easy work-up by an external magnet, and excellent product yield are the major advantages of the present methodology. A number of effective experimental parameters were also fully investigated to clear broadness and generality of the protocol.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Magnetic nanoparticle-supported eosin Y salt [SB-DABCO@eosin] as an efficient heterogeneous photocatalyst for the multi-component synthesis of chromeno[4,3-b]chromene in the presence of visible light.RSC Adv. 2022 Oct 11;12(45):28886-28901. doi: 10.1039/d2ra05122b. eCollection 2022 Oct 11. RSC Adv. 2022. PMID: 36320743 Free PMC article.
-
UV-visible light-induced photochemical synthesis of benzimidazoles by coomassie brilliant blue coated on W-ZnO@NH2 nanoparticles.RSC Adv. 2021 May 4;11(27):16359-16375. doi: 10.1039/d0ra10843j. eCollection 2021 Apr 30. RSC Adv. 2021. PMID: 35479136 Free PMC article.
-
An Efficient Synthesis of 2-Substituted Benzimidazoles via Photocatalytic Condensation of o-Phenylenediamines and Aldehydes.ACS Comb Sci. 2016 Oct 10;18(10):644-650. doi: 10.1021/acscombsci.6b00107. Epub 2016 Sep 26. ACS Comb Sci. 2016. PMID: 27631587
-
Diphenyl diselenide immobilized on magnetic nanoparticles: A novel and retrievable heterogeneous catalyst in the oxidation of aldehydes under mild and green conditions.J Colloid Interface Sci. 2018 Jan 1;509:485-494. doi: 10.1016/j.jcis.2017.09.034. Epub 2017 Sep 8. J Colloid Interface Sci. 2018. PMID: 28923746
-
Synthesis of Titania nanowires doped with Cd on the based Polycalix[4]resorcinarene for photocatalytic oxidation of aromatic alcohols under LED irradiation.Sci Rep. 2025 Mar 19;15(1):9400. doi: 10.1038/s41598-025-89742-2. Sci Rep. 2025. PMID: 40108194 Free PMC article.
Cited by
-
Magnetic nanoparticle-supported eosin Y salt [SB-DABCO@eosin] as an efficient heterogeneous photocatalyst for the multi-component synthesis of chromeno[4,3-b]chromene in the presence of visible light.RSC Adv. 2022 Oct 11;12(45):28886-28901. doi: 10.1039/d2ra05122b. eCollection 2022 Oct 11. RSC Adv. 2022. PMID: 36320743 Free PMC article.
-
Eco-Friendly Synthesis of 1H-benzo[d]imidazole Derivatives by ZnO NPs Characterization, DFT Studies, Antioxidant and Insilico Studies.Pharmaceuticals (Basel). 2023 Jul 6;16(7):969. doi: 10.3390/ph16070969. Pharmaceuticals (Basel). 2023. PMID: 37513881 Free PMC article.
-
TiO2/CuWO4 heterojunction photocatalyst in the preparation of cardiovascular chromeno[4,3-b]chromene drugs.RSC Adv. 2024 Dec 17;14(53):39636-39644. doi: 10.1039/d4ra07857h. eCollection 2024 Dec 10. RSC Adv. 2024. PMID: 39691223 Free PMC article.
-
UV-visible light-induced photochemical synthesis of benzimidazoles by coomassie brilliant blue coated on W-ZnO@NH2 nanoparticles.RSC Adv. 2021 May 4;11(27):16359-16375. doi: 10.1039/d0ra10843j. eCollection 2021 Apr 30. RSC Adv. 2021. PMID: 35479136 Free PMC article.
-
Facile one pot synthesis of 2-substituted benzimidazole derivatives under mild conditions by using engineered MgO@DFNS as heterogeneous catalyst.RSC Adv. 2023 Nov 1;13(46):32110-32125. doi: 10.1039/d3ra05761e. eCollection 2023 Oct 31. RSC Adv. 2023. PMID: 37920763 Free PMC article.
References
-
- Chen Y. Lu L. Q. Yu D. G. Zhu C. J. Xiao W. J. Sci. China: Chem. 2019;62(1):24–57. doi: 10.1007/s11426-018-9399-2. - DOI
-
- Xu S. Lu H. Chen L. Wang X. RSC Adv. 2014;4(85):45266–45274. doi: 10.1039/C4RA06632D. - DOI
-
- Kakhki R. M. Tayebee R. Ahsani F. J. Mater. Sci.: Mater. Electron. 2017;28(8):5941–5952. doi: 10.1007/s10854-016-6268-5. - DOI
-
- Mohammadzadeh Kakhki R. Tayebee R. Hedayat S. Appl. Organomet. Chem. 2018;32(2):e4033. doi: 10.1002/aoc.4033. - DOI
-
- Tayebee R. Mohammadzadeh Kakhki R. Audebert P. Amini M. M. Salehi M. Mahdizadeh Ghohe N. Mandanipour V. Karimipour G. R. Appl. Organomet. Chem. 2018;32(7):e4391. doi: 10.1002/aoc.4391. - DOI
LinkOut - more resources
Full Text Sources

