Transaminase-mediated synthesis of enantiopure drug-like 1-(3',4'-disubstituted phenyl)propan-2-amines
- PMID: 35519186
- PMCID: PMC9057730
- DOI: 10.1039/d0ra08134e
Transaminase-mediated synthesis of enantiopure drug-like 1-(3',4'-disubstituted phenyl)propan-2-amines
Abstract
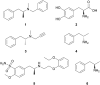
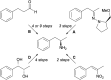
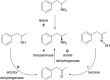
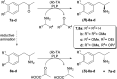
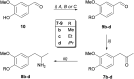
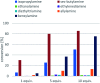
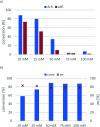
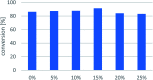
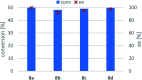
Transaminases (TAs) offer an environmentally and economically attractive method for the direct synthesis of pharmaceutically relevant disubstituted 1-phenylpropan-2-amine derivatives starting from prochiral ketones. In this work, we report the application of immobilised whole-cell biocatalysts with (R)-transaminase activity for the synthesis of novel disubstituted 1-phenylpropan-2-amines. After optimisation of the asymmetric synthesis, the (R)-enantiomers could be produced with 88-89% conversion and >99% ee, while the (S)-enantiomers could be selectively obtained as the unreacted fraction of the corresponding racemic amines in kinetic resolution with >48% conversion and >95% ee.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Stereoselective Access to 1-[2-Bromo(het)aryloxy]propan-2-amines Using Transaminases and Lipases; Development of a Chemoenzymatic Strategy Toward a Levofloxacin Precursor.J Org Chem. 2016 Oct 21;81(20):9765-9774. doi: 10.1021/acs.joc.6b01828. Epub 2016 Oct 7. J Org Chem. 2016. PMID: 27662230
-
Protein-engineering of an amine transaminase for the stereoselective synthesis of a pharmaceutically relevant bicyclic amine.Org Biomol Chem. 2016 Nov 2;14(43):10249-10254. doi: 10.1039/c6ob02139e. Org Biomol Chem. 2016. PMID: 27739550
-
Bioprospecting Reveals Class III ω-Transaminases Converting Bulky Ketones and Environmentally Relevant Polyamines.Appl Environ Microbiol. 2019 Jan 9;85(2):e02404-18. doi: 10.1128/AEM.02404-18. Print 2019 Jan 15. Appl Environ Microbiol. 2019. PMID: 30413473 Free PMC article.
-
omega-Transaminases for the synthesis of non-racemic alpha-chiral primary amines.Trends Biotechnol. 2010 Jun;28(6):324-32. doi: 10.1016/j.tibtech.2010.03.003. Epub 2010 Apr 27. Trends Biotechnol. 2010. PMID: 20430457 Review.
-
Features and technical applications of ω-transaminases.Appl Microbiol Biotechnol. 2012 Jun;94(5):1163-71. doi: 10.1007/s00253-012-4103-3. Epub 2012 May 4. Appl Microbiol Biotechnol. 2012. PMID: 22555915 Review.
Cited by
-
Non-Canonical Amino Acid-Based Engineering of (R)-Amine Transaminase.Front Chem. 2022 Feb 28;10:839636. doi: 10.3389/fchem.2022.839636. eCollection 2022. Front Chem. 2022. PMID: 35295971 Free PMC article.
References
-
- Ghislieri D. Turner N. J. Top. Catal. 2014;57:284–300. doi: 10.1007/s11244-013-0184-1. - DOI
-
- Nugent T. C., Chiral Amine Synthesis: Methods, Developments and Applications, Wiley-VCH, 2010
-
- Kim Y. J. Choi Y. S. Yang S. Yang W. R. Jeong J. H. Synlett. 2015;26:1981–1984. doi: 10.1055/s-0034-1380427. - DOI
-
- Hanson R. L. Davis B. L. Chen Y. Goldberg S. L. Parker W. L. Tully T. P. Montana M. A. Patel R. N. Adv. Synth. Catal. 2008;350:1367–1375. doi: 10.1002/adsc.200800084. - DOI
LinkOut - more resources
Full Text Sources

