Synthesis of new thienylpicolinamidine derivatives and possible mechanisms of antiproliferative activity
- PMID: 35519193
- PMCID: PMC9057764
- DOI: 10.1039/d0ra08796c
Synthesis of new thienylpicolinamidine derivatives and possible mechanisms of antiproliferative activity
Abstract
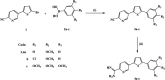
Three thienylpicolinamidine derivatives 4a-c were prepared from their corresponding picolinonitriles 3a-c on treatment with lithium trimethylsilylamide, LiN(TMS)2, followed by a de-protection step using ethanol/HCl (gas). DFT calculations were used to optimize the geometric structure of the newly synthesized picolinamidines. The comparison of DFT calculated spectral data with the experimental data (1H-NMR and 13C-NMR) showed a good agreement. The in vitro antiproliferative activity of the cationic compounds 4a-c was determined against 60 cancer cell lines representing nine types of cancer. The tested picolinamidines were highly active with compounds 4a and 4b eliciting mainly cytotoxic activity with GI values ranging from -7.17 to -86.03. Leukemia (SR and K-562), colon (SW-620 and HT29), and non-small cell lung cancer (NCI-H460) cell lines were the most responsive to the investigated picolinamidines. In particular, 4-methoxyphenyl derivative 4a showed a profound growth deterring power with GI50 of 0.34 μM against SR, 0.43 μM against SW-620, and 0.52 μM against NCI-H460. The three tested picolinamidines elicited potent GI50 values against all tested cell lines at low micromolar to sub-micromolar level. The new picolinamidines were selective and did not affect normal human fibroblasts. The selectivity index ranged from 13-21 μM. The novel picolinamidines downregulated the expression of key genes in the cell cycle, cdk1 and topoII, but did not affect p53 or txnrd1. Compounds 4b and 4c caused a significant reduction in the concentrations of TopoII and MAPK proteins but were devoid of any effect on the activity of caspase 3. Taken together, these promising anticancer candidates are effective at very low concentrations and safe to normal cells, and most probably work through arresting the cell cycle, and therefore, they deserve further investigations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Synthesis and antiproliferative activity of monocationic arylthiophene derivatives.Eur J Med Chem. 2017 Jan 27;126:789-798. doi: 10.1016/j.ejmech.2016.12.007. Epub 2016 Dec 2. Eur J Med Chem. 2017. PMID: 27951487
-
Synthesis, Antiproliferative Activity, and Apoptotic Profile of New Derivatives from the Meta Stable Benzoxazinone Scaffold.Anticancer Agents Med Chem. 2022;22(6):1226-1237. doi: 10.2174/1871520621666210706152632. Anticancer Agents Med Chem. 2022. PMID: 34229594
-
In vitro Antiproliferative Properties of Lipophililic-Acid Chelating Fluoroquinolones and TriazoloFluoroquinolones with 7-dihaloanilinosubstitution.Anticancer Agents Med Chem. 2022;22(19):3304-3321. doi: 10.2174/1871520622666220513154744. Anticancer Agents Med Chem. 2022. PMID: 35570520
-
Antiproliferative Properties of 7,8-Ethylene Diamine Chelator-Lipophilic Fluoroquinolone Derivatives Against Colorectal Cancer Cell Lines.Anticancer Agents Med Chem. 2022;22(5):1012-1028. doi: 10.2174/1871520621666210623111744. Anticancer Agents Med Chem. 2022. PMID: 34165411
-
Novel antiproliferative agents bearing substituted thieno[2,3-d]pyrimidine scaffold as dual VEGFR-2 and BRAF kinases inhibitors and apoptosis inducers; design, synthesis and molecular docking.Bioorg Chem. 2022 Aug;125:105861. doi: 10.1016/j.bioorg.2022.105861. Epub 2022 May 10. Bioorg Chem. 2022. PMID: 35569190
Cited by
-
Synthesis of inventive biphenyl and azabiphenyl derivatives as potential insecticidal agents against the cotton leafworm, Spodoptera littoralis.BMC Chem. 2023 Oct 27;17(1):144. doi: 10.1186/s13065-023-01050-w. BMC Chem. 2023. PMID: 37891573 Free PMC article.
References
-
- Thuita J. K. Wolf K. K. Murilla G. A. Bridges A. S. Boykin D. W. Mutuku J. N. Liu Q. Jones S. K. Gem C. O. Ching S. Tidwell R. R. Wang M. Z. Paine M. F. Brun R. Chemotherapy of Second Stage Human African Trypanosomiasis: Comparison between the Parenteral Diamidine DB829 and Its Oral Prodrug DB868 in Vervet Monkeys. PLoS Neglected Trop. Dis. 2015;9:e0003409. doi: 10.1371/journal.pntd.0003409. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

