Cherenkov light emission in molecular radiation therapy of the thyroid and its application to dosimetry
- PMID: 35519238
- PMCID: PMC9045923
- DOI: 10.1364/BOE.448139
Cherenkov light emission in molecular radiation therapy of the thyroid and its application to dosimetry
Abstract
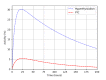
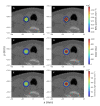
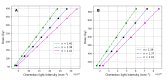
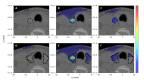
Numerical experiments based on Monte Carlo simulations and clinical CT data are performed to investigate the spatial and spectral characteristics of Cherenkov light emission and the relationship between Cherenkov light intensity and deposited dose in molecular radiotherapy of hyperthyroidism and papillary thyroid carcinoma. It is found that Cherenkov light is emitted mostly in the treatment volume, the spatial distribution of Cherenkov light at the surface of the patient presents high-value regions at locations that depend on the symmetry and location of the treatment volume, and the surface light in the near-infrared spectral region originates from the treatment site. The effect of inter-patient variability in the tissue optical parameters and radioisotope uptake on the linear relationship between the dose absorbed by the treatment volume and Cherenkov light intensity at the surface of the patient is investigated, and measurements of surface light intensity for which this effect is minimal are identified. The use of Cherenkov light measurements at the patient surface for molecular radiation therapy dosimetry is also addressed.
Published by Optica Publishing Group under the terms of the Creative Commons Attribution 4.0 License. Further distribution of this work must maintain attribution to the author(s) and the published article’s title, journal citation, and DOI.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Zhang R., Glaser A., Esipova T. V., Kanick S. C., Davis S. C., Vinogradov S., Gladstone D., Pogue B. W., “Čerenkov radiation emission and excited luminescence (crel) sensitivity during external beam radiation therapy: Monte carlo and tissue oxygenation phantom studies,” Biomed. Opt. Express 3(10), 2381–2394 (2012).10.1364/BOE.3.002381 - DOI - PMC - PubMed
-
- Čerenkov P., “Visible radiation produced by electrons moving in a medium with velocities exceeding that of light,” Phys. Rev. 52(4), 378 (1937).10.1103/PhysRev.52.378 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
