Lock-in functional near-infrared spectroscopy for measurement of the haemodynamic brain response
- PMID: 35519260
- PMCID: PMC9045899
- DOI: 10.1364/BOE.448038
Lock-in functional near-infrared spectroscopy for measurement of the haemodynamic brain response
Abstract
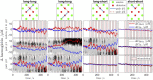
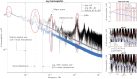
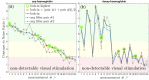
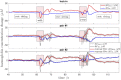
Here we show a method of the lock-in amplifying near-infrared signals originating within a human brain. It implies using two 90-degree rotated source-detector pairs fixed on a head surface. Both pairs have a joint sensitivity region located towards the brain. A direct application of the lock-in technique on both signals results in amplifying common frequency components, e.g. related to brain cortex stimulation and attenuating the rest, including all components not related to the stimulation: e.g. pulse, instrumental and biological noise or movement artefacts. This is a self-driven method as no prior assumptions are needed and the noise model is provided by the interfering signals themselves. We show the theory (classical modified Beer-Lambert law and diffuse optical tomography approaches), the algorithm implementation and tests on a finite element mathematical model and in-vivo on healthy volunteers during visual cortex stimulation. The proposed hardware and algorithm complexity suit the entire spectrum of (continuous wave, frequency domain, time-resolved) near-infrared spectroscopy systems featuring real-time, direct, robust and low-noise brain activity registration tool. As such, this can be of special interest in optical brain computer interfaces and high reliability/stability monitors of tissue oxygenation.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest related to this article.
Figures





Similar articles
-
PV-MBLL algorithm for extraction of absolute tissue oxygenation information by diffuse optical spectroscopy.Comput Methods Programs Biomed. 2020 Sep;193:105456. doi: 10.1016/j.cmpb.2020.105456. Epub 2020 Mar 17. Comput Methods Programs Biomed. 2020. PMID: 32305645
-
Comparison of functional activation responses from the auditory cortex derived using multi-distance frequency domain and continuous wave near-infrared spectroscopy.Neurophotonics. 2021 Oct;8(4):045004. doi: 10.1117/1.NPh.8.4.045004. Epub 2021 Dec 15. Neurophotonics. 2021. PMID: 34926716 Free PMC article.
-
Quantitative Comparison of Analytical Solution and Finite Element Method for Investigation of Near-infrared Light Propagation in Brain Tissue Model.Basic Clin Neurosci. 2023 Mar-Apr;14(2):193-202. doi: 10.32598/bcn.2021.1930.1. Epub 2023 Mar 1. Basic Clin Neurosci. 2023. PMID: 38107524 Free PMC article.
-
A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology.Neuroimage. 2014 Jan 15;85 Pt 1:6-27. doi: 10.1016/j.neuroimage.2013.05.004. Epub 2013 May 16. Neuroimage. 2014. PMID: 23684868 Review.
-
Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity.Neuroimage Clin. 2015 Mar 24;8:1-31. doi: 10.1016/j.nicl.2015.03.016. eCollection 2015. Neuroimage Clin. 2015. PMID: 26110109 Free PMC article. Review.
Cited by
-
Introduction to the Optics and the Brain 2023 feature issue.Biomed Opt Express. 2024 Mar 4;15(4):2110-2113. doi: 10.1364/BOE.517678. eCollection 2024 Apr 1. Biomed Opt Express. 2024. PMID: 38633102 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
