Blue-LIRIC in the rabbit cornea: efficacy, tissue effects, and repetition rate scaling
- PMID: 35519279
- PMCID: PMC9045900
- DOI: 10.1364/BOE.448286
Blue-LIRIC in the rabbit cornea: efficacy, tissue effects, and repetition rate scaling
Abstract
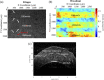
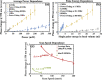
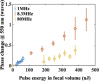
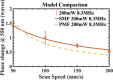
Laser-induced refractive index change (LIRIC) is being developed as a non-invasive way to alter optical properties of transparent, ophthalmic materials including corneas ex vivo and in vivo. This study examined the optical and biological effects of blue-LIRIC (wavelengths 400-405 nm) of ex-vivo rabbit corneas. Following LIRIC treatment at low and high repetition rates (8.3 MHz and 80 MHz, respectively), we interferometrically measured optical phase change, obtained transmission electron microscopy (TEM) micrographs, and stained histological sections with collagen hybridizing peptides (CHP) to assess the structural and organizational changes caused by LIRIC at different repetition rates. Finally, we performed power and scan speed scaling experiments at three different repetition rates (1 MHz, 8.3 MHz, and 80 MHz) to study their impact on LIRIC efficacy. Histologic co-localization of CHP and LIRIC-generated green autofluorescence signals suggested that collagen denaturation had occurred in the laser-irradiated region. TEM imaging showed different ultrastructural modifications for low and high repetition rate writing, with discrete homogenization of collagen fibrils at 80 MHz, as opposed to contiguous homogenization at 8.3 MHz. Overall, this study confirmed that LIRIC efficacy can be dramatically increased, while still avoiding tissue ablation, by lowering the repetition rate from 80 MHz to 8.3 MHz. Modeling suggests that this is due to a higher, single-pulse, energy density deposition at given laser powers during 8.3 MHz LIRIC.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
WHK has founder’s equity in Clerio Vision, and he is Chief Science Officer, but has no fiduciary or management responsibility. KRH has founder’s equity in Clerio Vision Inc., but no fiduciary responsibility. LZ is an employee of, and has equity in Clerio Vision Inc.
Figures





Similar articles
-
Multiphoton scaling of femtosecond laser-induced refractive index change (LIRIC) in hydrogels and rabbit cornea.Biomed Opt Express. 2024 Oct 8;15(11):6242-6258. doi: 10.1364/BOE.537705. eCollection 2024 Nov 1. Biomed Opt Express. 2024. PMID: 39553877 Free PMC article.
-
Effects of laser repetition rate on corneal tissue ablation for 193-nm excimer laser light.Lasers Surg Med. 2008 Sep;40(7):483-93. doi: 10.1002/lsm.20656. Lasers Surg Med. 2008. PMID: 18727026
-
Comparison of broadband and ultrabroadband pulses at MHz and GHz pulse-repetition rates for nonlinear femtosecond-laser scanning microscopy.J Biophotonics. 2011 Jan;4(1-2):84-91. doi: 10.1002/jbio.201000010. Epub 2010 Mar 10. J Biophotonics. 2011. PMID: 20222101
-
Acute ultrastructural changes of cornea after excimer laser ablation.Invest Ophthalmol Vis Sci. 1992 Mar;33(3):540-6. Invest Ophthalmol Vis Sci. 1992. PMID: 1544782
-
Effect of pulse to pulse interactions on ultra-short pulse laser drilling of steel with repetition rates up to 10 MHz.Opt Express. 2014 Jul 28;22(15):18790-9. doi: 10.1364/OE.22.018790. Opt Express. 2014. PMID: 25089496
Cited by
-
Multiphoton scaling of femtosecond laser-induced refractive index change (LIRIC) in hydrogels and rabbit cornea.Biomed Opt Express. 2024 Oct 8;15(11):6242-6258. doi: 10.1364/BOE.537705. eCollection 2024 Nov 1. Biomed Opt Express. 2024. PMID: 39553877 Free PMC article.
-
The Chemistry and Biology of Collagen Hybridization.J Am Chem Soc. 2023 May 24;145(20):10901-10916. doi: 10.1021/jacs.3c00713. Epub 2023 May 9. J Am Chem Soc. 2023. PMID: 37158802 Free PMC article. Review.
References
-
- Bashir Z. S., Ali M. H., Anwar A., Ayub M. H., Butt N. H., “Femto-LASIK: the recent innovation in laser assisted refractive surgery,” J. Pak. Med. Assoc. 67, 609 (2017). - PubMed
-
- Krueger R., Trokel S., Schubert H., “Interaction of ultraviolet laser light with the cornea,” Invest. Ophthalmol. Vis. Sci. 26, 1455–1464 (1985). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
