UV surface disinfection in a wearable drug delivery device
- PMID: 35519282
- PMCID: PMC9045911
- DOI: 10.1364/BOE.453270
UV surface disinfection in a wearable drug delivery device
Abstract
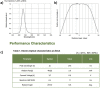
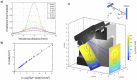
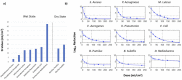
The advent of recombinant DNA technology fundamentally altered the drug discovery landscape, replacing traditional small-molecule drugs with protein and peptide-based biologics. Being susceptible to degradation via the oral route, biologics require comparatively invasive injections, most commonly by intravenous infusion (IV). Significant academic and industrial efforts are underway to replace IV transport with subcutaneous delivery by wearable infusion devices. To further complement the ease-of-use and safety of disposable infusion devices, surface disinfection of the drug container can be automated. For ease of use, the desired injector is a combination device, where the drug is inside the injector as a single solution combination device. The main obstacle of the desired solution is the inability to sterilize both injector and drug in the same chamber or using the same method (Gamma for the drug and ETO for the injector). This leads to the assembly of both drug container and injector after sterilization, resulting in at least one transition area that is not sterilized. To automate the delivery of the drug to the patient, a disinfection step before the drug delivery through the injector is required on the none-sterilized interface. As an innovative solution, the autoinjector presented here is designed with a single ultraviolet light-emitting diode (UV LED) for surface disinfection of the drug container and injector interface. In order to validate microbial disinfection similar to ethanol swabbing on the injector, a bacterial 3 or 6 log reduction needed to be demonstrated. However, the small disinfection chamber surfaces within the device are incapable of holding an initial bacterial load for demonstrating the 3 or 6 log reduction, complicating the validation method, and presenting a dilemma as to how to achieve the log reduction while producing real chamber conditions. The suggested solution in this paper is to establish a correlation model between the UV irradiance distribution within the disinfection chamber and a larger external test setup, which can hold the required bacterial load and represents a worse-case test scenario. Bacterial log reduction was subsequently performed on nine different microorganisms of low to high UV-tolerance. The procedure defined herein can be adopted for other surface or chamber disinfection studies in which the inoculation space is limited.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Alfaro J. A., Bohländer P., Dai M., Filius M., Howard C. J., van Kooten X. F., Ohayon S., Pomorski A., Schmid S., Aksimentiev A., Anslyn E. v., Bedran G., Cao C., Chinappi M., Coyaud E., Dekker C., Dittmar G., Drachman N., Eelkema R., Goodlett D., Hentz S., Kalathiya U., Kelleher N. L., Kelly R. T., Kelman Z., Kim S. H., Kuster B., Rodriguez-Larrea D., Lindsay S., Maglia G., Marcotte E. M., Marino J. P., Masselon C., Mayer M., Samaras P., Sarthak K., Sepiashvili L., Stein D., Wanunu M., Wilhelm M., Yin P., Meller A., Joo C., “The emerging landscape of single-molecule protein sequencing technologies,” Nat. Methods 18(6), 604–617 (2021). 10.1038/s41592-021-01143-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
