Synthesis of tetracyclic indolin-3-ones through Pd-catalyzed intramolecular deacetylative dearomatization of 3-acetoxy-indoles
- PMID: 35519305
- PMCID: PMC9064017
- DOI: 10.1039/c9ra02569c
Synthesis of tetracyclic indolin-3-ones through Pd-catalyzed intramolecular deacetylative dearomatization of 3-acetoxy-indoles
Abstract
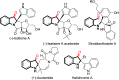
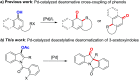
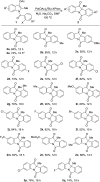
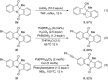
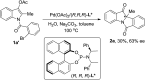
An efficient palladium-catalyzed intramolecular deacetylative dearomatization reaction of 3-acetoxyindoles has been developed. A range of tetracyclic indolin-3-ones bearing C2-quaternary stereocenters are achieved in good yields, showing a wide substrate scope for this reaction. A preliminary enantioselective reaction is established to furnish the product in 63% ee by using (R,R,R)-phosphoramide-PE as a chiral ligand.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Enantioselective Arylative Dearomatization of Indoles via Pd-Catalyzed Intramolecular Reductive Heck Reactions.J Am Chem Soc. 2015 Apr 22;137(15):4936-9. doi: 10.1021/jacs.5b01705. Epub 2015 Apr 10. J Am Chem Soc. 2015. PMID: 25849154
-
Dual catalysis for the redox annulation of nitroalkynes with indoles: enantioselective construction of indolin-3-ones bearing quaternary stereocenters.Angew Chem Int Ed Engl. 2015 Sep 14;54(38):11205-8. doi: 10.1002/anie.201504697. Epub 2015 Aug 5. Angew Chem Int Ed Engl. 2015. PMID: 26245887
-
Organocatalytic Stereodivergent Dearomatization and N-Acylation of 2-Amino-3-subsituted Indoles.Org Lett. 2024 Dec 20;26(50):10988-10992. doi: 10.1021/acs.orglett.4c04164. Epub 2024 Dec 5. Org Lett. 2024. PMID: 39635880
-
Oxidative Dearomatization of Indoles via Pd-Catalyzed C-H Oxygenation: An Entry to C2-Quaternary Indolin-3-ones.Org Lett. 2016 Apr 1;18(7):1534-7. doi: 10.1021/acs.orglett.6b00244. Epub 2016 Mar 17. Org Lett. 2016. PMID: 26986450
-
Palladium-Catalyzed Intramolecular Dearomatization of Indoles via Decarboxylative Alkynyl Termination.Org Lett. 2016 Aug 19;18(16):4016-9. doi: 10.1021/acs.orglett.6b01711. Epub 2016 Aug 10. Org Lett. 2016. PMID: 27508407
References
-
- Williams R. M. Glinka T. Kwast E. Coffman H. Stille J. K. J. Am. Chem. Soc. 1990;112:808. doi: 10.1021/ja00158a048. - DOI
- Liu J.-F. Jiang Z.-Y. Wang R.-R. Zeng Y.-T. Chen J.-J. Zhang X.-M. Ma Y.-B. Org. Lett. 2007;9:4127. doi: 10.1021/ol701540y. - DOI - PubMed
- Kumar C. V. S. Puranik V. G. Ramana C. V. Chem.–Eur. J. 2012;18:9601. doi: 10.1002/chem.201103604. - DOI - PubMed
- Abe T. Kukita A. Akiyama K. Naito T. Uemura D. Chem. Lett. 2012;41:728. doi: 10.1246/cl.2012.728. - DOI
- Gu W. Zhang Y. Hao X.-J. Yang F. M. Sun Q.-Y. Morris-Natschke S. L. Lee K.-H. Wang Y.-H. Long C.-L. J. Nat. Prod. 2014;77:2590. doi: 10.1021/np5003274. - DOI - PubMed
-
-
For selected recent examples:
- Yin Q. You S.-L. Chem. Sci. 2011;2:1344. doi: 10.1039/C1SC00190F. - DOI
- Rueping M. Raja S. Núñez A. Adv. Synth. Catal. 2011;353:563. doi: 10.1002/adsc.201000952. - DOI
- Parra A. Alfaro R. Marzo L. Moreno-Carrasco A. Garcia Ruano J. L. Aleman J. Chem. Commun. 2012;48:9759. doi: 10.1039/C2CC34053D. - DOI - PubMed
- Zhao Y.-L. Wang Y. Cao J. Liang Y.-M. Xu P.-F. Org. Lett. 2014;16:2438. doi: 10.1021/ol5008185. - DOI - PubMed
- Liu R.-R. Ye S.-C. Lu C.-J. Zhuang G.-L. Gao J.-R. Jia Y.-X. Angew. Chem., Int. Ed. 2015;54:11205. doi: 10.1002/anie.201504697. - DOI - PubMed
- Li J.-S. Liu Y.-J. Li S. Ma J.-A. Chem. Commun. 2018;54:9151. doi: 10.1039/C8CC05125A. - DOI - PubMed
- Li P. Yong W. Sheng R. Rao W. Zhu X. Zhang X. Adv. Synth. Catal. 2019;361:201. doi: 10.1002/adsc.201801162. - DOI
-
-
-
For recent reviews:
- Chen Q.-A. Ye Z.-S. Duan Y. Zhou Y.-G. Chem. Soc. Rev. 2013;42:497. doi: 10.1039/C2CS35333D. - DOI - PubMed
- Ding Q. Zhou X. Fan R. Org. Biomol. Chem. 2014;12:4807. doi: 10.1039/C4OB00371C. - DOI - PubMed
- Zhuo C.-X. Cheng C. You S.-L. Acc. Chem. Res. 2014;47:2558. doi: 10.1021/ar500167f. - DOI - PubMed
- Denizot N. Tomakinian T. Beaud R. Kouklovsky C. Vincent G. Tetrahedron Lett. 2015;56:4413. doi: 10.1016/j.tetlet.2015.05.078. - DOI
- Chen W. T. Zhang L. You S.-L. Chem. Soc. Rev. 2016;45:1570. doi: 10.1039/C5CS00356C. - DOI - PubMed
- Sun W. Li G. Hong L. Wang R. Org. Biomol. Chem. 2016;14:2164. doi: 10.1039/C5OB02526E. - DOI - PubMed
-
-
-
For selected recent examples:
- Bedford R. B. Fey N. Haddow M. F. Sankey R. F. Chem. Commun. 2011;47:3649. doi: 10.1039/C0CC05033D. - DOI - PubMed
- Wu K.-J. Dai L.-X. You S.-L. Org. Lett. 2012;14:3772. doi: 10.1021/ol301663h. - DOI - PubMed
- Yin B. Cai C. Zeng G. Zhang R. Li X. Jiang H. Org. Lett. 2012;14:1098. doi: 10.1021/ol300008d. - DOI - PubMed
- Wu K.-J. Dai L.-X. You S.-L. Chem. Commun. 2013;49:8620. doi: 10.1039/C3CC44631J. - DOI - PubMed
- Hata K. He Z. Daniliuc C. G. Itami K. Studer A. Chem. Commun. 2014;50:463. doi: 10.1039/C3CC47350C. - DOI - PubMed
- Ramella V. He Z. Daniliuc C. G. Studer A. Eur. J. Org. Chem. 2016:2268. doi: 10.1002/ejoc.201600194. - DOI
- Lei X. Xie H.-Y. Xu C. Liu X. Wen X. Sun H. Xu Q.-L. Adv. Synth. Catal. 2016;358:1892. doi: 10.1002/adsc.201600135. - DOI
- Liu J. Peng H. Lu L. Xu X. Jiang H. Yin B. Org. Lett. 2016;18:6440. doi: 10.1021/acs.orglett.6b03339. - DOI - PubMed
- Yang P. You S.-L. Org. Lett. 2018;20:7684. doi: 10.1021/acs.orglett.8b03425. - DOI - PubMed
-
-
- Zhao L. Li Z. Chang L. Xu J. Yao H. Wu X. Org. Lett. 2012;14:2066. doi: 10.1021/ol300584m. - DOI - PubMed
- Gao S. Yang C. Huang Y. Zhao L. Wu X. Yao H. Lin A. Org. Biomol. Chem. 2016;14:840. doi: 10.1039/C5OB01970B. - DOI - PubMed
- Douki K. Ono H. Taniguchi T. Shimokawa J. Kitamura M. Fukuyama T. J. Am. Chem. Soc. 2016;138:14578. doi: 10.1021/jacs.6b10237. - DOI - PubMed
- Li X. Zhou B. Yang R.-Z. Yang F.-M. Liang R.-X. Liu R.-R. Jia Y.-X. J. Am. Chem. Soc. 2018;140:13945. doi: 10.1021/jacs.8b09186. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

