A step-wise synthetic approach is necessary to access γ-conjugates of folate: folate-conjugated prodigiosenes
- PMID: 35519339
- PMCID: PMC9064012
- DOI: 10.1039/c9ra01435g
A step-wise synthetic approach is necessary to access γ-conjugates of folate: folate-conjugated prodigiosenes
Abstract
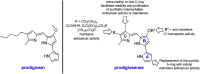
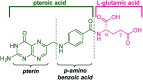
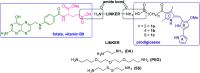
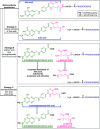
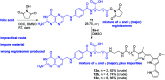
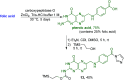
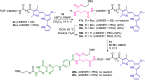
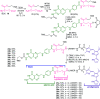
Despite the vast literature that describes reacting folic acid with a pharmacophore, this route is ineffective in providing the correct regioisomer of the resulting conjugate. We herein present a step-wise route to the preparation of nine folate conjugates of the tripyrrolic prodigiosene skeleton. The strict requirement for step-wise construction of the folate core is demonstrated, so as to achieve conjugation at only the desired γ-carboxylic acid and thus maintain the α-carboxylic site for folate receptor (FRα) recognition. Linkages via ethylenediamine, polyethylene glycol and glutathione are demonstrated.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict to declare.
Figures


Similar articles
-
Reactive Oxygen Species and Folate Receptor-Targeted Nanophotosensitizers Composed of Folic Acid-Conjugated and Poly(ethylene glycol)-Chlorin e6 Tetramer Having Diselenide Linkages for Targeted Photodynamic Treatment of Cancer Cells.Int J Mol Sci. 2022 Mar 14;23(6):3117. doi: 10.3390/ijms23063117. Int J Mol Sci. 2022. PMID: 35328538 Free PMC article.
-
"Click and go": simple and fast folic acid conjugation.Org Biomol Chem. 2014 May 28;12(20):3181-90. doi: 10.1039/c4ob00150h. Epub 2014 Apr 10. Org Biomol Chem. 2014. PMID: 24723199
-
Reduced 18F-Folate Conjugates as a New Class of PET Tracers for Folate Receptor Imaging.Bioconjug Chem. 2018 Apr 18;29(4):1119-1130. doi: 10.1021/acs.bioconjchem.7b00775. Epub 2018 Feb 19. Bioconjug Chem. 2018. PMID: 29412638
-
The folate receptor as a rational therapeutic target for personalized cancer treatment.Drug Resist Updat. 2014 Oct-Dec;17(4-6):89-95. doi: 10.1016/j.drup.2014.10.002. Epub 2014 Oct 8. Drug Resist Updat. 2014. PMID: 25457975 Review.
-
The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander?Int J Cancer. 2006 Jul 15;119(2):243-50. doi: 10.1002/ijc.21712. Int J Cancer. 2006. PMID: 16453285 Review.
Cited by
-
Mono-amino acid linkers enable highly potent small molecule-drug conjugates by conditional release.Mol Ther. 2024 Apr 3;32(4):1048-1060. doi: 10.1016/j.ymthe.2024.02.020. Epub 2024 Feb 17. Mol Ther. 2024. PMID: 38369752 Free PMC article.
-
Folic acid functionalization for targeting self-assembled paclitaxel-based nanoparticles.RSC Adv. 2022 Dec 12;12(54):35484-35493. doi: 10.1039/d2ra06306a. eCollection 2022 Dec 6. RSC Adv. 2022. PMID: 36544466 Free PMC article.
-
Functionalized in Triplicate: A Ring-By-Ring Approach to Tailored Prodiginine Derivatives for Site-Specific Conjugation Through Click Chemistry.Chemistry. 2025 Aug 1;31(43):e202502066. doi: 10.1002/chem.202502066. Epub 2025 Jul 14. Chemistry. 2025. PMID: 40626904 Free PMC article.
References
LinkOut - more resources
Full Text Sources

