A step-wise synthetic approach is necessary to access γ-conjugates of folate: folate-conjugated prodigiosenes
- PMID: 35519339
- PMCID: PMC9064012
- DOI: 10.1039/c9ra01435g
A step-wise synthetic approach is necessary to access γ-conjugates of folate: folate-conjugated prodigiosenes
Abstract
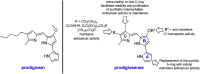
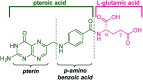
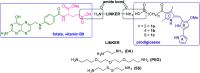
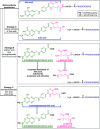
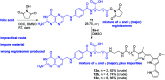
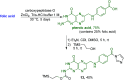
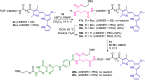
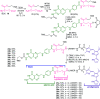
Despite the vast literature that describes reacting folic acid with a pharmacophore, this route is ineffective in providing the correct regioisomer of the resulting conjugate. We herein present a step-wise route to the preparation of nine folate conjugates of the tripyrrolic prodigiosene skeleton. The strict requirement for step-wise construction of the folate core is demonstrated, so as to achieve conjugation at only the desired γ-carboxylic acid and thus maintain the α-carboxylic site for folate receptor (FRα) recognition. Linkages via ethylenediamine, polyethylene glycol and glutathione are demonstrated.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict to declare.
Figures


References
LinkOut - more resources
Full Text Sources

