Efficient and sustainable laccase-catalyzed iodination of p-substituted phenols using KI as iodine source and aerial O2 as oxidant
- PMID: 35519358
- PMCID: PMC9065379
- DOI: 10.1039/c9ra02541c
Efficient and sustainable laccase-catalyzed iodination of p-substituted phenols using KI as iodine source and aerial O2 as oxidant
Abstract
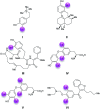
The laccase-catalyzed iodination of p-hydroxyarylcarbonyl- and p-hydroxyarylcarboxylic acid derivatives using KI as iodine source and aerial oxygen as the oxidant delivers the corresponding iodophenols in a highly efficient and sustainable manner with yields up to 93% on a preparative scale under mild reaction conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Wang L. Zhou X. Fredimoses M. Liao S. Liu Y. RSC Adv. 2014;4:57350–57376. doi: 10.1039/C4RA09833A. - DOI
- Gribble G. W., Naturally Occurring Organohalogen Compounds - A Comprehensive Update, Springer, Wien, 2010
-
- Aiello A. Fattorusso E. Imperatore C. Menna M. Müller W. E. G. Mar. Drugs. 2010;8:285–291. doi: 10.3390/md8020285. - DOI - PMC - PubMed
- Prawat H. Mahidol C. Kaweetripob W. Wittayalai S. Ruchirawat S. Tetrahedron. 2012;68:6881–6886. doi: 10.1016/j.tet.2012.06.018. - DOI
- Iizuka T. Fudou R. Jojima Y. Ogawa S. Yamanaka S. Inukai Y. Ojika M. J. Antibiot. 2006;59:385–391. doi: 10.1038/ja.2006.55. - DOI - PubMed
- Brent G. A. J. Clin. Invest. 2012;122:3035–3043. doi: 10.1172/JCI60047. - DOI - PMC - PubMed
-
-
For reviews, see:
- Roy D. Uozumi Y. Adv. Synth. Catal. 2018;360:602–625. doi: 10.1002/adsc.201700810. - DOI
- de Meijere A., Bräse S. and Oestreich M., Metal-Catalyzed Cross-Coupling Reactions and More, Wiley-VCH, Weinheim, 2014
- Johansson Seechurn C. C. C. Kitching M. O. Colacot T. J. Snieckus V. Angew. Chem., Int. Ed. 2012;51:5062–5085. doi: 10.1002/anie.201107017. - DOI - PubMed
- Nicolaou K. C. Bulger P. G. Sarlah D. Angew. Chem., Int. Ed. 2005;44:4442–4489. doi: 10.1002/anie.200500368. - DOI - PubMed
-
-
- Wang R. Mo S. Lu Y. Shen Z. Adv. Synth. Catal. 2011;353:713–718. doi: 10.1002/adsc.201000730. - DOI
- Miao H. Yang Z. Org. Lett. 2000;2:1765–1768. doi: 10.1021/ol000087t. - DOI - PubMed
- Ross A. J. Lang H. L. Jackson R. F. W. J. Org. Chem. 2010;75:245–248. doi: 10.1021/jo902238n. - DOI - PubMed
- Iranpoor N. Firouzabadi H. Safavi A. Motevalli S. Doroodmand M. M. Appl. Organomet. Chem. 2012;26:417–424. doi: 10.1002/aoc.2868. - DOI
- Takami K. Yorimitsu H. Shinokubo H. Matsubara S. Oshima K. Org. Lett. 2001;3:1997–1999. doi: 10.1021/ol015975i. - DOI - PubMed
- Pacardo D. B. Sethi M. Jones S. E. Naik R. R. Knecht M. R. ACS Nano. 2009;3:1288–1296. doi: 10.1021/nn9002709. - DOI - PubMed
- Singh C. Prakasham A. P. Gangwar M. K. Butcher R. J. Ghosh P. ACS Omega. 2018;3:1740–1756. doi: 10.1021/acsomega.7b01974. - DOI - PMC - PubMed
- Fernandes T. d. A. Gontijo Vaz B. Eberlin M. N. da Silva A. J. M. Costa P. R. R. J. Org. Chem. 2010;75:7085–7091. doi: 10.1021/jo1010922. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

