Overview of protein posttranslational modifications in Arthropoda venoms
- PMID: 35519418
- PMCID: PMC9036706
- DOI: 10.1590/1678-9199-JVATITD-2021-0047
Overview of protein posttranslational modifications in Arthropoda venoms
Abstract
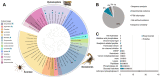
Accidents with venomous animals are a public health issue worldwide. Among the species involved in these accidents are scorpions, spiders, bees, wasps, and other members of the phylum Arthropoda. The knowledge of the function of proteins present in these venoms is important to guide diagnosis, therapeutics, besides being a source of a large variety of biotechnological active molecules. Although our understanding about the characteristics and function of arthropod venoms has been evolving in the last decades, a major aspect crucial for the function of these proteins remains poorly studied, the posttranslational modifications (PTMs). Comprehension of such modifications can contribute to better understanding the basis of envenomation, leading to improvements in the specificities of potential therapeutic toxins. Therefore, in this review, we bring to light protein/toxin PTMs in arthropod venoms by accessing the information present in the UniProtKB/Swiss-Prot database, including experimental and putative inferences. Then, we concentrate our discussion on the current knowledge on protein phosphorylation and glycosylation, highlighting the potential functionality of these modifications in arthropod venom. We also briefly describe general approaches to study "PTM-functional-venomics", herein referred to the integration of PTM-venomics with a functional investigation of PTM impact on venom biology. Furthermore, we discuss the bottlenecks in toxinology studies covering PTM investigation. In conclusion, through the mining of PTMs in arthropod venoms, we observed a large gap in this field that limits our understanding on the biology of these venoms, affecting the diagnosis and therapeutics development. Hence, we encourage community efforts to draw attention to a better understanding of PTM in arthropod venom toxins.
Keywords: Arthropod venom; Glycosylation; Mass spectrometry-based proteomics; PTM-functional-venomics; PTM-venomics; Phosphorylation; Posttranslational modification; UniProtKB/Swiss-Prot database.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Brasil Acidente por animais peçonhentos: Notificações registradas no Sistema de Informação de Agravos de Notificação. DATASUS. 2021 http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/animaisbr.def
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
