Establishment of atropisomerism in 3-indolyl furanoids: a synthetic, experimental and theoretical perspective
- PMID: 35519481
- PMCID: PMC9066644
- DOI: 10.1039/c9ra05350f
Establishment of atropisomerism in 3-indolyl furanoids: a synthetic, experimental and theoretical perspective
Abstract
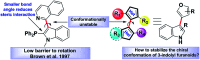
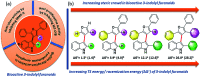
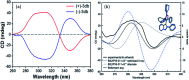
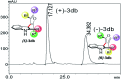
Introduction of axial chirality in bioactive 3-indolyl furanoids has been achieved by systematic alteration of functional groups around the stereogenic axis, keeping in mind that atropisomerically pure analogues may possess different binding affinities and selectivities towards a target protein. The kinetics of racemization of axially chiral 3-indolyl furanoids have been studied through chiral HPLC analysis, electronic circular dichroism (ECD) spectroscopy, and computational modeling. The results identify the configurational parameters for optically pure 3-indolyl furanoids to exist as stable and isolable atropisomeric form.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Eliel E. L. and Wilen S. H., Stereochemistry of Organic Compounds, Wiley, New York, 1994
-
- Bringmann G. Gulder T. Gulder T. A. M. Breuning M. Chem. Rev. 2011;111:563. doi: 10.1021/cr100155e. - DOI - PubMed
- Qin T. Joiner S. L. S. Khalil Z. G. Johnson R. P. Capon R. J. Porco Jr J. A. Nat. Chem. 2015;7:234. doi: 10.1038/nchem.2173. - DOI - PMC - PubMed
- Bringmann G. Menche D. Acc. Chem. Res. 2001;34:615. doi: 10.1021/ar000106z. - DOI - PubMed
- Baudoin O. Gueritte F. Stud. Nat. Prod. Chem. 2003;29:355.
- Qin T. Joiner S. L. S. Khalil Z. G. Johnson R. P. Capon R. J. Porco Jr J. A. Nat. Chem. 2015;7:234. doi: 10.1038/nchem.2173. - DOI - PMC - PubMed
- Clayden J. Moran W. J. Edwards P. J. LaPlante S. R. Angew. Chem., Int. Ed. 2009;48:6398. doi: 10.1002/anie.200901719. - DOI - PubMed
- Qiu L. Wu J. Chan S. A-Yeung T. T.-L. Ji J. X. Guo R. Pai C. C. Zhou Z. Li X. Fan Q. H. Chan A. S. C. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5815. doi: 10.1073/pnas.0307774101. - DOI - PMC - PubMed
-
- Zask A. Murphy J. Ellestad G. A. Chirality. 2013;25:265. doi: 10.1002/chir.22145. - DOI - PubMed
- Takahashi H. Wakamatsu S. Tabata H. Oshitari T. Harada A. Inoue K. Natsugari H. Org. Lett. 2011;13:4760. - PubMed
- Smith D. E. Marquez I. Lokensgard M. E. Rheingold A. L. Hecht D. A. Gustafson J. L. Angew. Chem., Int. Ed. 2015;54:11754. doi: 10.1002/anie.201506085. - DOI - PubMed
-
- Jaisankar P., Swarnakar S., Chatterjee S., Verma S., Mandal M., and Chaudhuri S. R., US Pat., US20180230135, 2018
-
Unpublished results.
-
- Zhang S. Yao Q. J. Liao G. Li X. Li H. Chen H. M. Hong X. Shi B. F. ACS Catal. 2019;9:1956. doi: 10.1021/acscatal.8b04870. - DOI
- Raut V. S. Jean M. Vanthuyne N. Roussel C. Constantieux T. Bressy C. Bugaut X. Bonne D. Rodriguez J. J. Am. Chem. Soc. 2017;139:2140. doi: 10.1021/jacs.6b11079. - DOI - PubMed
- Cardoso F. S. P. Abboud K. A. Aponick A. J. Am. Chem. Soc. 2013;135:14548. doi: 10.1021/ja407689a. - DOI - PubMed
- Alkorta I. Elguero J. Roussel C. Vanthuyne N. Piras P. Adv. Heterocycl. Chem. 2012;105:1. doi: 10.1016/B978-0-12-396530-1.00001-2. - DOI
- Bonne D. Rodriguez J. Chem. Commun. 2017;53:12385. doi: 10.1039/C7CC06863H. - DOI - PubMed
- Bonne D. Rodriguez J. Eur. J. Org. Chem. 2018:2417. doi: 10.1002/ejoc.201800078. - DOI
LinkOut - more resources
Full Text Sources

