Preparation of new adsorbent-supported Fe/Ni particles for the removal of crystal violet and methylene blue by a heterogeneous Fenton-like reaction
- PMID: 35519486
- PMCID: PMC9066707
- DOI: 10.1039/c9ra04710g
Preparation of new adsorbent-supported Fe/Ni particles for the removal of crystal violet and methylene blue by a heterogeneous Fenton-like reaction
Abstract
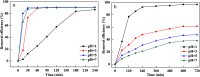
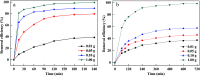
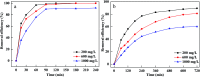
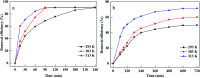
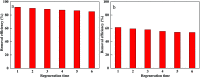
Prepared material-supported Fe/Ni particles (PM-Fe/Ni) were produced and applied as an adsorbent, reductant and Fenton-like catalyst for removing methylene blue (MB) and crystal violet (CV) from aqueous solutions. Fe/Ni particles were prepared by reducing ferric chloride with sodium borohydride and supported on the produced porous material. Various techniques including X-ray diffraction (XRD), Brunauer-Emmett-Teller (BET), Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy analysis (SEM) were employed to characterize the crystal phase, surface area, surface morphology and functional groups. Removal experiments were conducted to study the effects of different factors such as PM-Fe/Ni dosage, initial pH, H2O2 concentration, initial concentrations and temperature on MB and CV removal. The removal efficiency of CV and MB by PM-Fe/Ni/H2O2 were 91.86% and 61.41% under the conditions of dye concentration of 1000 mg L-1, H2O2 concentration of 50 mM, PM-Fe/Ni dosage of 0.20 g and temperature of 293 K. The analysis of the degradation kinetics showed that the degradation of MB and CV followed well pseudo-first-order kinetics. A possible mechanism of removal of MB and CV was proposed, including the adsorption, reduction and dominating Fenton oxidation. The regeneration experiments of PM-Fe/Ni demonstrated that PM-Fe/Ni with H2O2 still showed a high removal efficiency after six reaction cycles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Wu J. Gao H. Yao S. Chen L. Gao Y. Zhang H. Sep. Purif. Technol. 2015;147:179–185. doi: 10.1016/j.seppur.2015.04.022. - DOI
-
- Chakraborty S. Chowdhury S. Saha P. D. Carbohydr. Polym. 2011;86:1533–1541. doi: 10.1016/j.carbpol.2011.06.058. - DOI
-
- Ambika S. Devasena M. Nambi I. M. Chem. Eng. J. 2017;181:847–855. - PubMed
-
- Chen S. Wu Y. Li G. Wu J. Meng G. Guo X. Liu Z. Appl. Clay Sci. 2017;136:103–111. doi: 10.1016/j.clay.2016.11.016. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

