An eco-friendly route for template-free synthesis of high specific surface area mesoporous CeO2 powders and their adsorption for acid orange 7
- PMID: 35519489
- PMCID: PMC9066840
- DOI: 10.1039/c9ra02294e
An eco-friendly route for template-free synthesis of high specific surface area mesoporous CeO2 powders and their adsorption for acid orange 7
Abstract
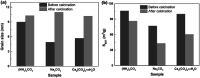
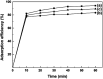
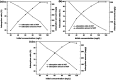
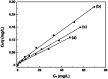
An eco-friendly route was developed for the synthesis of mesoporous CeO2 powders without any additional template. The original cerium precursors were separated from Ce3+ aqueous solution by (NH4)2CO3 or Na2CO3 via a chemical precipitation method, then H2O2 was introduced to induce the phase transformation from original cerium precursors to CeO2 precursors with initial porous structures, finally the crystallinities of CeO2 precursors were improved by a hydrothermal treatment, meanwhile the mesoporous structures of final CeO2 powders were formed. The BET surface areas of mesoporous CeO2 powders synthesized using (NH4)2CO3 and Na2CO3 as precipitants were 106.1 and 76.9 m2 g-1, respectively. Moreover, a mesoporous CeO2 sample with BET surface area of 100.0 m2 g-1 was also synthesized using commercial Ce2(CO3)3·xH2O as an existing cerium precursor under the same conditions as control, which could shorten experimental processes and reduce costs. The oxidation-induced phase transformation from original cerium precursors to CeO2 precursors with initial porous structures was the precondition for further forming of mesoporous structures of final CeO2 powders during the hydrothermal process. These mesoporous CeO2 powders showed the rapid and effective adsorption for acid orange 7 dye from simulated wastewater without pH pre-adjustment at room temperature. Furthermore, the adsorption capacities of these mesoporous CeO2 powders for removal of acid orange 7 dye were determined according to the Langmuir linear fits.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Effective and Efficient Porous CeO2 Adsorbent for Acid Orange 7 Adsorption.Materials (Basel). 2023 Mar 27;16(7):2650. doi: 10.3390/ma16072650. Materials (Basel). 2023. PMID: 37048943 Free PMC article.
-
Oxidation-Induced and Hydrothermal-Assisted Template-Free Synthesis of Mesoporous CeO2 for Adsorption of Acid Orange 7.Materials (Basel). 2022 Jul 27;15(15):5209. doi: 10.3390/ma15155209. Materials (Basel). 2022. PMID: 35955142 Free PMC article.
-
Sm doped mesoporous CeO2 nanocrystals: aqueous solution-based surfactant assisted low temperature synthesis, characterization and their improved autocatalytic activity.Dalton Trans. 2016 Jan 28;45(4):1679-92. doi: 10.1039/c5dt03688g. Epub 2015 Dec 24. Dalton Trans. 2016. PMID: 26699084
-
Study of Mesostructured CeO2 Synthesis via Nanocasting Using SBA-15 as a Template: Influence of the Cerium Precursor.Int J Mol Sci. 2024 Dec 3;25(23):13016. doi: 10.3390/ijms252313016. Int J Mol Sci. 2024. PMID: 39684726 Free PMC article.
-
Catalytic wet peroxide degradation of acrylonitrile wastewater by ordered mesoporous Ag/CeO2: synthesis, performance and kinetics.RSC Adv. 2021 Apr 29;11(26):15959-15968. doi: 10.1039/d1ra01258d. eCollection 2021 Apr 26. RSC Adv. 2021. PMID: 35481213 Free PMC article.
Cited by
-
Effective and Efficient Porous CeO2 Adsorbent for Acid Orange 7 Adsorption.Materials (Basel). 2023 Mar 27;16(7):2650. doi: 10.3390/ma16072650. Materials (Basel). 2023. PMID: 37048943 Free PMC article.
-
Building Ohmic Contact Interfaces toward Ultrastable Zn Metal Anodes.Adv Sci (Weinh). 2021 Dec;8(23):e2102612. doi: 10.1002/advs.202102612. Epub 2021 Oct 20. Adv Sci (Weinh). 2021. PMID: 34672109 Free PMC article.
-
Surface basicity mediated rapid and selective adsorptive removal of Congo red over nanocrystalline mesoporous CeO2.Nanoscale Adv. 2021 Sep 21;3(23):6704-6718. doi: 10.1039/d1na00412c. eCollection 2021 Nov 24. Nanoscale Adv. 2021. PMID: 36132658 Free PMC article.
-
Atomic Layer Deposition of CeO2 Film with a Novel Heteroleptic Ce(III) Complex.Molecules. 2024 Jun 23;29(13):2987. doi: 10.3390/molecules29132987. Molecules. 2024. PMID: 38998939 Free PMC article.
-
Oxidation-Induced and Hydrothermal-Assisted Template-Free Synthesis of Mesoporous CeO2 for Adsorption of Acid Orange 7.Materials (Basel). 2022 Jul 27;15(15):5209. doi: 10.3390/ma15155209. Materials (Basel). 2022. PMID: 35955142 Free PMC article.
References
-
- Vinodgopal K. Peller J. Makogon O. Kamat P. V. Water Res. 1998;32:3646–3650. doi: 10.1016/S0043-1354(98)00154-7. - DOI
-
- Liu L. Y. Pu M. Yang L. Evans D. G. He J. Mater. Chem. Phys. 2007;106:422–427. doi: 10.1016/j.matchemphys.2007.06.022. - DOI
-
- Daneshvar E. Kousha M. Koutahzadeh N. Sohrabi M. S. Bhatnagar A. Environ. Prog. Sustainable Energy. 2013;32:285–293. doi: 10.1002/ep.11623. - DOI
-
- Vinodgopal K. Peller J. Makogon O. Kamat P. V. Water Res. 1998;32:3646–3650. doi: 10.1016/S0043-1354(98)00154-7. - DOI
LinkOut - more resources
Full Text Sources

