Utilization of metabolic energy in treatment of ocular surface disorders: polyphosphate as an energy source for corneal epithelial cell proliferation
- PMID: 35519495
- PMCID: PMC9066647
- DOI: 10.1039/c9ra04409d
Utilization of metabolic energy in treatment of ocular surface disorders: polyphosphate as an energy source for corneal epithelial cell proliferation
Abstract
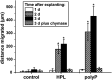
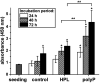
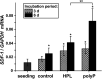
Impaired regeneration of the corneal epithelium, as found in many ocular surface diseases, is a major clinical problem in ophthalmology. We hypothesized that corneal epithelial regeneration can be promoted by the physiological, energy-delivering as well as "morphogenetically active" polymer, inorganic polyphosphate (polyP). Corneal limbal explants (diameter, 4 mm) were cultivated on collagen-coated well plates in the absence or presence of polyP (chain length, ∼40 Pi units; 50 μg ml-1) or human platelet lysate (hp-lysate; 5% v/v). Cell outgrowth and differentiation were analyzed after staining with DRAQ5 (nuclei) and rhodamine phalloidin (cytoskeleton), as well as by environmental scanning electron microscopy (ESEM). Cell growth/viability of hCECs was assessed by XTT assay. The expression of SDF-1 was quantitated by qRT-PCR. Exposure to hp-lysate (also containing polyP) increased cell migration already at day 1. Even stronger was the effect of polyP. This effect was blocked by a mast cell serine protease. The formation of cell multilayers was enhanced by hp-lysate or even more by polyP. ESEM revealed continuous cell junctions and prominent microvilli on the surface of adjacent cells exposed to polyP; those structures were only rarely seen in the controls. The hp-lysate and, more potently, polyP increased the proliferation of hCECs, as well as SDF-1 expression. The findings indicate the potential usefulness of the natural polymer, polyP, for topical treatment of corneal epithelial defects. Future studies are directed to develop suitable formulations of polyP, such as biomimetic polyP nano/microparticles showing an adjustable release kinetics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Polyphosphate, the physiological metabolic fuel for corneal cells: a potential biomaterial for ocular surface repair.Biomater Sci. 2019 Dec 1;7(12):5506-5515. doi: 10.1039/c9bm01289c. Epub 2019 Oct 31. Biomater Sci. 2019. PMID: 31667480
-
3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone.Acta Biomater. 2017 Dec;64:377-388. doi: 10.1016/j.actbio.2017.09.031. Epub 2017 Sep 28. Acta Biomater. 2017. PMID: 28966095
-
Collagen-inducing biologization of prosthetic material for hernia repair: Polypropylene meshes coated with polyP/collagen.J Biomed Mater Res B Appl Biomater. 2018 Aug;106(6):2109-2121. doi: 10.1002/jbm.b.34016. Epub 2017 Oct 10. J Biomed Mater Res B Appl Biomater. 2018. PMID: 29024311
-
Polyphosphate: A Morphogenetically Active Implant Material Serving as Metabolic Fuel for Bone Regeneration.Macromol Biosci. 2015 Sep;15(9):1182-97. doi: 10.1002/mabi.201500100. Epub 2015 May 15. Macromol Biosci. 2015. PMID: 25982003 Review.
-
Characterization, isolation, expansion and clinical therapy of human corneal epithelial stem/progenitor cells.J Stem Cells. 2014;9(2):79-91. J Stem Cells. 2014. PMID: 25158157 Review.
Cited by
-
Biomimetic Polyphosphate Materials: Toward Application in Regenerative Medicine.Prog Mol Subcell Biol. 2022;61:83-130. doi: 10.1007/978-3-031-01237-2_5. Prog Mol Subcell Biol. 2022. PMID: 35697938
-
Anti-Fibrotic Effect of SDF-1β Overexpression in Bleomycin-Injured Rat Lung.Pharmaceutics. 2022 Aug 27;14(9):1803. doi: 10.3390/pharmaceutics14091803. Pharmaceutics. 2022. PMID: 36145551 Free PMC article.
References
-
- Thoft R. A. Friend J. Invest. Ophthalmol. Visual Sci. 1983;24:1442–1443. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

