Metal- and photocatalyst-free synthesis of 3-selenylindoles and asymmetric diarylselenides promoted by visible light
- PMID: 35519497
- PMCID: PMC9067025
- DOI: 10.1039/c9ra03642c
Metal- and photocatalyst-free synthesis of 3-selenylindoles and asymmetric diarylselenides promoted by visible light
Abstract
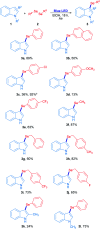
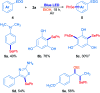
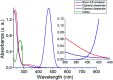
A novel and sustainable procedure was developed for the synthesis of 3-selenylindoles employing diorganyl diselenides and indoles or electron-rich arenes as starting materials. Visible blue light was used to promote the reaction without employing transition metal complexes or organic photocatalysts as sensitizers. Additives such as strong oxidants or bases were not required. Moreover, ethanol was employed as a benign solvent under mild reaction conditions. Through this easy and eco-friendly approach, several 3-selenylindoles and a number of asymmetric diarylselenides were obtained in good to excellent isolated yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
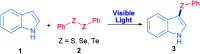
Similar articles
-
Visible-Light-Induced, Graphene Oxide-Promoted C3-Chalcogenylation of Indoles Strategy under Transition-Metal-Free Conditions.Molecules. 2022 Jan 25;27(3):772. doi: 10.3390/molecules27030772. Molecules. 2022. PMID: 35164036 Free PMC article.
-
Visible light-promoted photocatalyst-free activation of persulfates: a promising strategy for C-H functionalization reactions.Org Biomol Chem. 2022 Apr 20;20(16):3249-3262. doi: 10.1039/d2ob00109h. Org Biomol Chem. 2022. PMID: 35363233 Review.
-
Visible-light induced selenocyclization of 2-ethynylanilines under ambient conditions: simple FeBr3 as a dual-functional catalyst.Org Biomol Chem. 2024 Jul 31;22(30):6198-6204. doi: 10.1039/d4ob01062k. Org Biomol Chem. 2024. PMID: 39028029
-
A solvent- and metal-free synthesis of 3-chacogenyl-indoles employing DMSO/I2 as an eco-friendly catalytic oxidation system.J Org Chem. 2014 May 2;79(9):4125-30. doi: 10.1021/jo5000779. Epub 2014 Apr 18. J Org Chem. 2014. PMID: 24712301
-
The formation reaction of a carbon-carbon bond promoted by Eosin-Y under visible light.Org Biomol Chem. 2025 Apr 16;23(16):3741-3799. doi: 10.1039/d5ob00141b. Org Biomol Chem. 2025. PMID: 40159809 Review.
Cited by
-
Recent Advances in Light-Induced Selenylation.ACS Org Inorg Au. 2022 Aug 19;2(6):455-463. doi: 10.1021/acsorginorgau.2c00033. eCollection 2022 Dec 7. ACS Org Inorg Au. 2022. PMID: 36855533 Free PMC article. Review.
-
Dimsyl Anion Enables Visible-Light-Promoted Charge Transfer in Cross-Coupling Reactions of Aryl Halides.Adv Synth Catal. 2022 Jan 18;364(2):420-425. doi: 10.1002/adsc.202101052. Epub 2021 Oct 15. Adv Synth Catal. 2022. PMID: 37197314 Free PMC article.
-
A diselenide additive enables photocatalytic hydroalkoxylation of gem-difluoroalkenes.Chem Commun (Camb). 2023 May 4;59(37):5623-5626. doi: 10.1039/d3cc01012k. Chem Commun (Camb). 2023. PMID: 37082905 Free PMC article.
-
CoFe2O4/Cu(OH)2 magnetic nanocomposite: an efficient and reusable heterogeneous catalyst for one-pot synthesis of β-hydroxy-1,4-disubstituted-1,2,3-triazoles from epoxides.RSC Adv. 2019 Sep 20;9(51):29873-29887. doi: 10.1039/c9ra06038c. eCollection 2019 Sep 18. RSC Adv. 2019. PMID: 35531545 Free PMC article.
-
Synthetic strategies for aryl/heterocyclic selenides and tellurides under transition-metal-catalyst free conditions.RSC Adv. 2021 Feb 10;11(12):6682-6698. doi: 10.1039/d0ra10629a. eCollection 2021 Feb 4. RSC Adv. 2021. PMID: 35423206 Free PMC article. Review.
References
-
- Mugesh G. du Mont W.-W. Sies H. Chem. Rev. 2001;101:2125. doi: 10.1021/cr000426w. - DOI - PubMed
- Bhabak K. P. Mugesh G. Acc. Chem. Res. 2010;43:1408. doi: 10.1021/ar100059g. - DOI - PubMed
- Kumar S. Yan J. Poon J.-f. Singh V. P. Lu X. Ott M. K. Engman L. Kumar S. Angew. Chem., Int. Ed. 2016;55:3729. doi: 10.1002/anie.201510947. - DOI - PubMed
- Lenardão E. J., Santi C. and Sancineto L., in Organoselenium Compounds, Springer, 2018, p. 99
- Nogueira C. W. Zeni G. Rocha J. B. T. Chem. Rev. 2004;104:6255. doi: 10.1021/cr0406559. - DOI - PubMed
-
- Manjare S. T. Kim Y. Churchill D. G. Acc. Chem. Res. 2014;47:2985. doi: 10.1021/ar500187v. - DOI - PubMed
- Rampon D. S. Rodembusch F. S. Schneider J. M. F. M. Bechto l. H. Gonçalves P. F. B. Merlo A. A. Schneider P. H. J. Mater. Chem. 2010;20:715. doi: 10.1039/B917366H. - DOI
- Goswami S. Hazra A. Chakrabarty R. Fun H.-K. Org. Lett. 2009;11:4350. doi: 10.1021/ol901737s. - DOI - PubMed
- Tang B. Xing Y. Li P. Zhang N. Yu F. Yang G. J. Am. Chem. Soc. 2007;129:11666. doi: 10.1021/ja072572q. - DOI - PubMed
-
- Back T. G., in Organoselenium Chemistry: A Practical Approach, Oxford University Press, Oxford, 1999
- Freudendahl D. M. Santoro S. Shahzad S. A. Santi C. Wirth T. Angew. Chem., Int. Ed. 2009;48:8409. doi: 10.1002/anie.200903893. - DOI - PubMed
- Mukherjee A. J. Zade S. S. Singh H. B. Sunoj R. B. Chem. Rev. 2010;110:4357. doi: 10.1021/cr900352j. - DOI - PubMed
- Godoi M. Paixão M. W. Braga A. L. Dalton Trans. 2011;40:11347. doi: 10.1039/C1DT11022E. - DOI - PubMed
- Santoro S. Azeredo J. B. Nascimento V. Sancineto L. Braga A. L. Santi C. RSC Adv. 2014;4:31521. doi: 10.1039/C4RA04493B. - DOI
LinkOut - more resources
Full Text Sources

