Metal- and photocatalyst-free synthesis of 3-selenylindoles and asymmetric diarylselenides promoted by visible light
- PMID: 35519497
- PMCID: PMC9067025
- DOI: 10.1039/c9ra03642c
Metal- and photocatalyst-free synthesis of 3-selenylindoles and asymmetric diarylselenides promoted by visible light
Abstract
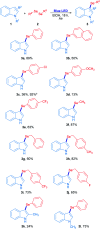
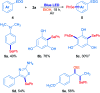
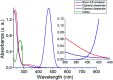
A novel and sustainable procedure was developed for the synthesis of 3-selenylindoles employing diorganyl diselenides and indoles or electron-rich arenes as starting materials. Visible blue light was used to promote the reaction without employing transition metal complexes or organic photocatalysts as sensitizers. Additives such as strong oxidants or bases were not required. Moreover, ethanol was employed as a benign solvent under mild reaction conditions. Through this easy and eco-friendly approach, several 3-selenylindoles and a number of asymmetric diarylselenides were obtained in good to excellent isolated yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
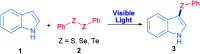
References
-
- Mugesh G. du Mont W.-W. Sies H. Chem. Rev. 2001;101:2125. doi: 10.1021/cr000426w. - DOI - PubMed
- Bhabak K. P. Mugesh G. Acc. Chem. Res. 2010;43:1408. doi: 10.1021/ar100059g. - DOI - PubMed
- Kumar S. Yan J. Poon J.-f. Singh V. P. Lu X. Ott M. K. Engman L. Kumar S. Angew. Chem., Int. Ed. 2016;55:3729. doi: 10.1002/anie.201510947. - DOI - PubMed
- Lenardão E. J., Santi C. and Sancineto L., in Organoselenium Compounds, Springer, 2018, p. 99
- Nogueira C. W. Zeni G. Rocha J. B. T. Chem. Rev. 2004;104:6255. doi: 10.1021/cr0406559. - DOI - PubMed
-
- Manjare S. T. Kim Y. Churchill D. G. Acc. Chem. Res. 2014;47:2985. doi: 10.1021/ar500187v. - DOI - PubMed
- Rampon D. S. Rodembusch F. S. Schneider J. M. F. M. Bechto l. H. Gonçalves P. F. B. Merlo A. A. Schneider P. H. J. Mater. Chem. 2010;20:715. doi: 10.1039/B917366H. - DOI
- Goswami S. Hazra A. Chakrabarty R. Fun H.-K. Org. Lett. 2009;11:4350. doi: 10.1021/ol901737s. - DOI - PubMed
- Tang B. Xing Y. Li P. Zhang N. Yu F. Yang G. J. Am. Chem. Soc. 2007;129:11666. doi: 10.1021/ja072572q. - DOI - PubMed
-
- Back T. G., in Organoselenium Chemistry: A Practical Approach, Oxford University Press, Oxford, 1999
- Freudendahl D. M. Santoro S. Shahzad S. A. Santi C. Wirth T. Angew. Chem., Int. Ed. 2009;48:8409. doi: 10.1002/anie.200903893. - DOI - PubMed
- Mukherjee A. J. Zade S. S. Singh H. B. Sunoj R. B. Chem. Rev. 2010;110:4357. doi: 10.1021/cr900352j. - DOI - PubMed
- Godoi M. Paixão M. W. Braga A. L. Dalton Trans. 2011;40:11347. doi: 10.1039/C1DT11022E. - DOI - PubMed
- Santoro S. Azeredo J. B. Nascimento V. Sancineto L. Braga A. L. Santi C. RSC Adv. 2014;4:31521. doi: 10.1039/C4RA04493B. - DOI
LinkOut - more resources
Full Text Sources

