Detection of streptavidin-biotin intermediate metastable states at the single-molecule level using high temporal-resolution atomic force microscopy
- PMID: 35519498
- PMCID: PMC9067134
- DOI: 10.1039/c9ra04106k
Detection of streptavidin-biotin intermediate metastable states at the single-molecule level using high temporal-resolution atomic force microscopy
Abstract
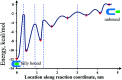
Although the streptavidin-biotin intermolecular bond has been extensively used in many applications due to its high binding affinity, its exact nature and interaction mechanism have not been well understood. Several reports from previous studies gave a wide range of results in terms of the system's energy potential landscape because of bypassing some short-lived states in the detection process. We employed a quasi-static process of slowly loading force onto the bond (loading rate = 20 pN s-1) to minimize the force-induced disruption and to provide a chance to explore the system in near-equilibrium. Therein, by utilizing a fast sampling rate for the detection of force by atomic force microscopy (20 μs per data point), several transient states of the system were clearly resolved in our force spectroscopy measurements. These key strategies allow the determination of the states' relative positions and free energy levels along the pulling reaction coordinate for the illustration of an energy landscape of the system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Monodisperse measurement of the biotin-streptavidin interaction strength in a well-defined pulling geometry.PLoS One. 2017 Dec 5;12(12):e0188722. doi: 10.1371/journal.pone.0188722. eCollection 2017. PLoS One. 2017. PMID: 29206886 Free PMC article.
-
Energy landscape of streptavidin-biotin complexes measured by atomic force microscopy.Biochemistry. 2000 Aug 22;39(33):10219-23. doi: 10.1021/bi992715o. Biochemistry. 2000. PMID: 10956011
-
Energy landscape roughness of the streptavidin-biotin interaction.J Mol Recognit. 2007 Nov-Dec;20(6):495-501. doi: 10.1002/jmr.841. J Mol Recognit. 2007. PMID: 17902095
-
Toward mechanical manipulations of cell membranes and membrane proteins using an atomic force microscope: an invited review.Cell Biochem Biophys. 2003;39(3):257-77. doi: 10.1385/CBB:39:3:257. Cell Biochem Biophys. 2003. PMID: 14716080 Review.
-
Force as a single molecule probe of multidimensional protein energy landscapes.Curr Opin Struct Biol. 2013 Feb;23(1):48-57. doi: 10.1016/j.sbi.2012.11.007. Epub 2012 Dec 30. Curr Opin Struct Biol. 2013. PMID: 23279960 Review.
Cited by
-
Dendronized oligoethylene glycols with phosphonate tweezers for cell-repellent coating of oxide surfaces: coarse-scale and nanoscopic interfacial forces.RSC Adv. 2021 May 17;11(29):17727-17733. doi: 10.1039/d1ra02571f. eCollection 2021 May 13. RSC Adv. 2021. PMID: 35480187 Free PMC article.
-
Quantifying molecular- to cellular-level forces in living cells.J Phys D Appl Phys. 2021 Dec 2;54(48):483001. doi: 10.1088/1361-6463/ac2170. Epub 2021 Sep 9. J Phys D Appl Phys. 2021. PMID: 34866655 Free PMC article.
References
-
- Diamandis E. P. Christopoulos T. K. Clin. Chem. 1991;37:625–636. - PubMed
LinkOut - more resources
Full Text Sources

