Constitutional isomers of brominated-functionalized copillar[5]arenes: synthesis, characterization, and crystal structures
- PMID: 35519554
- PMCID: PMC9063920
- DOI: 10.1039/c9ra02313e
Constitutional isomers of brominated-functionalized copillar[5]arenes: synthesis, characterization, and crystal structures
Abstract
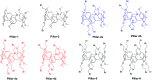
We herein report the preparation of constitutional isomers of brominated-functionalized pillar[5]arenes via co-condensation of 1,4-bis(2-bromoethoxy)benzene and 1,4-dimethoxybenzene. The structures of the obtained isomers were then established using single crystal X-ray diffraction. We also found that the isomeric yield distribution of the different constitutional isomers was independent of the monomer's mole feed ratio, as revealed by HPLC analysis of the crude mixture. Finally, further characterization of the separated constitutional isomers indicated that they possess different melting points, NMR spectra, crystal structures, binding constants and stacking patterns in the solid state.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Constitutional Isomers of Pentahydroxy-Functionalized Pillar[5]arenes: Synthesis, Characterization, and Crystal Structures.J Org Chem. 2017 Oct 20;82(20):10945-10952. doi: 10.1021/acs.joc.7b01837. Epub 2017 Oct 9. J Org Chem. 2017. PMID: 28960074
-
Efficient synthesis of copillar[5]arenes and their host-guest properties with dibromoalkanes.Org Biomol Chem. 2011 Oct 21;9(20):7007-10. doi: 10.1039/c1ob05871a. Epub 2011 Aug 25. Org Biomol Chem. 2011. PMID: 21870001
-
Two 2 : 3 copillar[5]arene constitutional isomers: syntheses, crystal structures and host-guest complexation of their derivatives with dicarboxylic acid sodium salts in water.Chem Commun (Camb). 2013 Feb 4;49(11):1070-2. doi: 10.1039/c2cc38355a. Chem Commun (Camb). 2013. PMID: 23282481
-
Pillar[5]arenes: fascinating cyclophanes with a bright future.Chem Soc Rev. 2012 Jan 21;41(2):597-607. doi: 10.1039/c1cs15164a. Epub 2011 Jul 29. Chem Soc Rev. 2012. PMID: 21804967 Review.
-
Noncovalently bound and mechanically interlocked systems using pillar[n]arenes.Chem Soc Rev. 2022 May 10;51(9):3648-3687. doi: 10.1039/d2cs00169a. Chem Soc Rev. 2022. PMID: 35445234 Review.
Cited by
-
Crystal structure and supra-molecular features of a bis-urea-functionalized pillar[5]arene.Acta Crystallogr E Crystallogr Commun. 2023 Oct 19;79(Pt 11):1044-1048. doi: 10.1107/S2056989023009003. eCollection 2023 Nov 1. Acta Crystallogr E Crystallogr Commun. 2023. PMID: 37936859 Free PMC article.
-
Spatially Designed Supramolecular Anion Receptors Based on Pillar[5]arene Scaffolds: Synthesis and Halide Anion Binding Properties.ACS Omega. 2022 Dec 27;8(1):1466-1475. doi: 10.1021/acsomega.2c06903. eCollection 2023 Jan 10. ACS Omega. 2022. PMID: 36643541 Free PMC article.
References
-
- Han C. Ma F. Zhang Z. Xia B. Yu Y. Huang F. Org. Lett. 2010;12:4360–4363. doi: 10.1021/ol1018344. - DOI - PubMed
- Cao D. Kou Y. Liang J. Chen Z. Wang L. Meier H. Angew. Chem., Int. Ed. 2009;48:9721–9723. doi: 10.1002/anie.200904765. - DOI - PubMed
- Liu L. Cao D. Jin Y. Tao H. Koua Y. Meier H. Org. Biomol. Chem. 2011;9:7007–7010. doi: 10.1039/C1OB05871A. - DOI - PubMed
-
- Li Z. Yang J. He J. Abliz Z. Huang F. Org. Lett. 2014;16:2065–2069. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

