Nanohybrid layered double hydroxide materials as efficient catalysts for methanol electrooxidation
- PMID: 35519556
- PMCID: PMC9063938
- DOI: 10.1039/c9ra01270b
Nanohybrid layered double hydroxide materials as efficient catalysts for methanol electrooxidation
Abstract
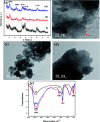
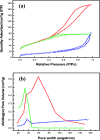
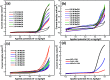
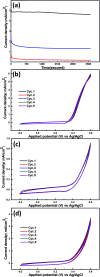
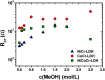
In this work, efficient methanol oxidation fuel cell catalysts with excellent stability in alkaline media have been synthesized by including transition metals to the layered double hydroxide (LDH) nanohybrids. The nanohybrids CoCr-LDH, NiCoCr-LDH and NiCr-LDH were prepared by co-precipitation and their physicochemical characteristics were investigated using TEM, XRD, IR and BET analyses. The nanohybrid CoCr-LDH is found to have the highest surface area of 179.87 m2 g-1. The electrocatalytic activity measurements showed that the current density was increased by increasing the methanol concentration (from 0.1 to 3 M) as a result of its increased oxidation at the surface. The nanohybrid NiCr-LDH, showing the highest pore size (55.5 Å) showed the highest performance for methanol oxidation, with a current density of 7.02 mA cm-2 at 60 mV s-1 using 3 M methanol. In addition, the corresponding onset potential was 0.35 V (at 60 mV s-1 using 3 M methanol) which is the lowest value among all other used LDH nanohybrids. Overall, we observed the following reactivity order: NiCr-LDH > NiCoCr-LDH > CoCr-LDH, as derived from the impedance spectroscopy analysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Flower-like Layered NiCu-LDH/MXene Nanocomposites as an Anodic Material for Electrocatalytic Oxidation of Methanol.Langmuir. 2023 Apr 4;39(13):4756-4765. doi: 10.1021/acs.langmuir.3c00154. Epub 2023 Mar 21. Langmuir. 2023. PMID: 36943685
-
Valorization of spent double substituted Co-Ni-Zn-Fe LDH wastewater nanoadsorbent as methanol electro-oxidation catalyst.Sci Rep. 2022 Nov 11;12(1):19354. doi: 10.1038/s41598-022-23798-2. Sci Rep. 2022. PMID: 36369455 Free PMC article.
-
Synthesis of nickel-based layered double hydroxide (LDH) and their adsorption on carbon felt fibres: application as low cost cathode catalyst in microbial fuel cell (MFC).Environ Technol. 2021 Jan;42(3):492-504. doi: 10.1080/09593330.2019.1635652. Epub 2019 Jul 16. Environ Technol. 2021. PMID: 31223060
-
Applications of Mechanochemically Prepared Layered Double Hydroxides as Adsorbents and Catalysts: A Mini-Review.Nanomaterials (Basel). 2019 Jan 8;9(1):80. doi: 10.3390/nano9010080. Nanomaterials (Basel). 2019. PMID: 30626167 Free PMC article. Review.
-
Layered double hydroxide based materials applied in persulfate based advanced oxidation processes: Property, mechanism, application and perspectives.J Hazard Mater. 2022 Feb 15;424(Pt C):127612. doi: 10.1016/j.jhazmat.2021.127612. Epub 2021 Nov 8. J Hazard Mater. 2022. PMID: 34838358 Review.
Cited by
-
Multifunctional ternary ZnMgFe LDH as an efficient adsorbent for ceftriaxone sodium and antimicrobial agent: sustainability of adsorption waste as a catalyst for methanol electro-oxidation.RSC Adv. 2023 Sep 1;13(37):26069-26088. doi: 10.1039/d3ra03426g. eCollection 2023 Aug 29. RSC Adv. 2023. PMID: 37664207 Free PMC article.
-
Advantage of Dimethyl Sulfoxide in the Fabrication of Binder-Free Layered Double Hydroxides Electrodes: Impacts of Physical Parameters on the Crystalline Domain and Electrochemical Performance.Int J Mol Sci. 2022 Sep 5;23(17):10192. doi: 10.3390/ijms231710192. Int J Mol Sci. 2022. PMID: 36077588 Free PMC article.
-
DNA-Modified Cobalt Tungsten Oxide Hydroxide Hydrate Nanochains as an Effective Electrocatalyst with Amplified CO Tolerance during Methanol Oxidation.ACS Omega. 2021 Jul 13;6(29):19162-19169. doi: 10.1021/acsomega.1c02515. eCollection 2021 Jul 27. ACS Omega. 2021. PMID: 34337254 Free PMC article.
-
One-Step Synthesis of a Binder-Free, Stable, and High-Performance Electrode; Cu-O|Cu3P Heterostructure for the Electrocatalytic Methanol Oxidation Reaction (MOR).Nanomaterials (Basel). 2023 Mar 30;13(7):1234. doi: 10.3390/nano13071234. Nanomaterials (Basel). 2023. PMID: 37049328 Free PMC article.
-
A Facile Approach of Fabricating Bifunctional Catalysts for Redox Applications by Uniformly Immobilized Metallic Nanoparticles on NiCr LDH.Nanomaterials (Basel). 2023 Mar 9;13(6):987. doi: 10.3390/nano13060987. Nanomaterials (Basel). 2023. PMID: 36985881 Free PMC article.
References
-
- Habibi B. Ghaderi S. Electrosynthesized Ni-Al Layered Double Hydroxide-Pt Nanoparticles as an Inorganic Nanocomposite and Potentate Anodic Material for Methanol Electrooxidation in Alkaline Media. Bull. Chem. React. Eng. Catal. 2017;12:1–13. doi: 10.9767/bcrec. doi: 10.9767/bcrec.12.1.460.1-13. - DOI - DOI
-
- Zhao S. Yan L. Luo H. Mustain W. Xu H. Recent Progress and Perspectives of Bifunctional Oxygen Reduction/Evolution Catalyst Development for Unitized Regenerative Anion Exchange Membrane Fuel Cells. Nano Energy. 2018;47:172–198. doi: 10.1016/j.nanoen.2018.02.015. doi: 10.1016/j.nanoen.2018.02.015. - DOI - DOI
-
- Huang W. Wang H. Zhou J. Wang J. Duchesne P. N. Muir D. Zhang P. Han N. Zhao F. Zeng M. Zhong J. Jin C. Li Y. Lee S. T. Dai H. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum-nickel hydroxide-graphene. Nat. Commun. 2015;6:1–8. doi: 10.1038/ncomms10035. - DOI - PMC - PubMed
-
- Khouchaf A. Takky D. Chbihi M. E. M. Benmokhtar S. Electrocatalytic oxidation of methanol on glassy carbon electrode modified by metal ions (copper and nickel) dispersed into polyaniline film. J. Mater. Sci. Chem. Eng. 2016;4:97–105. doi: 10.4236/msce.2016.42011. - DOI
LinkOut - more resources
Full Text Sources

