Nanohybrid layered double hydroxide materials as efficient catalysts for methanol electrooxidation
- PMID: 35519556
- PMCID: PMC9063938
- DOI: 10.1039/c9ra01270b
Nanohybrid layered double hydroxide materials as efficient catalysts for methanol electrooxidation
Abstract
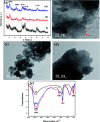
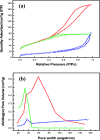
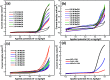
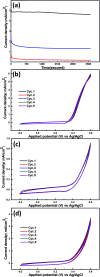
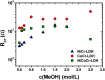
In this work, efficient methanol oxidation fuel cell catalysts with excellent stability in alkaline media have been synthesized by including transition metals to the layered double hydroxide (LDH) nanohybrids. The nanohybrids CoCr-LDH, NiCoCr-LDH and NiCr-LDH were prepared by co-precipitation and their physicochemical characteristics were investigated using TEM, XRD, IR and BET analyses. The nanohybrid CoCr-LDH is found to have the highest surface area of 179.87 m2 g-1. The electrocatalytic activity measurements showed that the current density was increased by increasing the methanol concentration (from 0.1 to 3 M) as a result of its increased oxidation at the surface. The nanohybrid NiCr-LDH, showing the highest pore size (55.5 Å) showed the highest performance for methanol oxidation, with a current density of 7.02 mA cm-2 at 60 mV s-1 using 3 M methanol. In addition, the corresponding onset potential was 0.35 V (at 60 mV s-1 using 3 M methanol) which is the lowest value among all other used LDH nanohybrids. Overall, we observed the following reactivity order: NiCr-LDH > NiCoCr-LDH > CoCr-LDH, as derived from the impedance spectroscopy analysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Habibi B. Ghaderi S. Electrosynthesized Ni-Al Layered Double Hydroxide-Pt Nanoparticles as an Inorganic Nanocomposite and Potentate Anodic Material for Methanol Electrooxidation in Alkaline Media. Bull. Chem. React. Eng. Catal. 2017;12:1–13. doi: 10.9767/bcrec. doi: 10.9767/bcrec.12.1.460.1-13. - DOI - DOI
-
- Zhao S. Yan L. Luo H. Mustain W. Xu H. Recent Progress and Perspectives of Bifunctional Oxygen Reduction/Evolution Catalyst Development for Unitized Regenerative Anion Exchange Membrane Fuel Cells. Nano Energy. 2018;47:172–198. doi: 10.1016/j.nanoen.2018.02.015. doi: 10.1016/j.nanoen.2018.02.015. - DOI - DOI
-
- Huang W. Wang H. Zhou J. Wang J. Duchesne P. N. Muir D. Zhang P. Han N. Zhao F. Zeng M. Zhong J. Jin C. Li Y. Lee S. T. Dai H. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum-nickel hydroxide-graphene. Nat. Commun. 2015;6:1–8. doi: 10.1038/ncomms10035. - DOI - PMC - PubMed
-
- Khouchaf A. Takky D. Chbihi M. E. M. Benmokhtar S. Electrocatalytic oxidation of methanol on glassy carbon electrode modified by metal ions (copper and nickel) dispersed into polyaniline film. J. Mater. Sci. Chem. Eng. 2016;4:97–105. doi: 10.4236/msce.2016.42011. - DOI
LinkOut - more resources
Full Text Sources

