NiO decorated CeO2 nanostructures as room temperature isopropanol gas sensors
- PMID: 35519582
- PMCID: PMC9063907
- DOI: 10.1039/c9ra00441f
NiO decorated CeO2 nanostructures as room temperature isopropanol gas sensors
Abstract
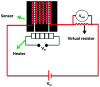
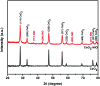
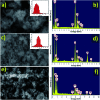
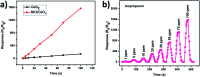
Heterostructures developed using CeO2 show promising peculiarities in the field of metal oxide gas sensors due to the great variations in the resistance during the adsorption and desorption processes. NiO decorated CeO2 nanostructures (NiO/CeO2) were synthesized via a facile two-step process. High resolution transmission electron microscopy (HRTEM) results revealed the perfect decoration of NiO on the CeO2 surface. The porous nature of the NiO/CeO2 sensor surface was confirmed from scanning electron microscopy (SEM) analysis. Gas sensing studies of pristine CeO2 and NiO/CeO2 sensors were performed under room conditions and enhanced gas sensing properties for the NiO/CeO2 sensor towards isopropanol were observed. Decoration of NiO on the CeO2 surface develops a built-in potential at the interface of NiO and CeO2 which played a vital role in the superior sensing performance of the NiO/CeO2 sensor. Sharp response and recovery times (15 s/19 s) were observed for the NiO/CeO2 sensor towards 100 ppm isopropanol at room temperature. Long-term stability of the NiO/CeO2 sensor was also studied and discussed. From all the results it is concluded that the decoration of NiO on the CeO2 surface could significantly enhance the sensing performance and it has great advantages in designing best performing isopropanol gas sensors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Development of high-performance sensor based on NiO/SnO2 heterostructures to study sensing properties towards various reducing gases.Nanotechnology. 2020 Sep 25;31(39):395502. doi: 10.1088/1361-6528/ab98bb. Epub 2020 Jun 2. Nanotechnology. 2020. PMID: 32485683
-
In situ deposited hierarchical CuO/NiO nanowall arrays film sensor with enhanced gas sensing performance to H2S.J Hazard Mater. 2020 Mar 5;385:121570. doi: 10.1016/j.jhazmat.2019.121570. Epub 2019 Nov 8. J Hazard Mater. 2020. PMID: 31753669
-
MoS2-NiO nanocomposite for H2S sensing at room temperature.RSC Adv. 2023 Sep 29;13(41):28564-28575. doi: 10.1039/d3ra05241a. eCollection 2023 Sep 26. RSC Adv. 2023. PMID: 37780733 Free PMC article.
-
Noble Metal-Decorated Nanostructured Zinc Oxide: Strategies to Advance Chemiresistive Hydrogen Gas Sensing.Chem Rec. 2022 Jul;22(7):e202200090. doi: 10.1002/tcr.202200090. Epub 2022 Jun 14. Chem Rec. 2022. PMID: 35703683 Review.
-
Nickel Oxide Nanostructures for Gas Sensing: Recent Advances, Challenges, and Future Perspectives.ACS Sens. 2025 Mar 28;10(3):1641-1674. doi: 10.1021/acssensors.4c02946. Epub 2025 Mar 10. ACS Sens. 2025. PMID: 40059852 Review.
Cited by
-
Facile synthesis of NiFe2O4-based nanoblocks for low-temperature detection of trace n-butanol.RSC Adv. 2024 Jan 10;14(4):2214-2225. doi: 10.1039/d3ra07264a. eCollection 2024 Jan 10. RSC Adv. 2024. PMID: 38213961 Free PMC article.
-
Synthesis of ((CeO2)0.8(Sm2O3)0.2)@NiO Core-Shell Type Nanostructures and Microextrusion Printing of a Composite Anode Based on Them.Materials (Basel). 2022 Dec 13;15(24):8918. doi: 10.3390/ma15248918. Materials (Basel). 2022. PMID: 36556722 Free PMC article.
-
Graphene Oxide Thin Films for Detection and Quantification of Industrially Relevant Alcohols and Acetic Acid.Sensors (Basel). 2023 Jan 1;23(1):462. doi: 10.3390/s23010462. Sensors (Basel). 2023. PMID: 36617058 Free PMC article.
-
ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment.Micromachines (Basel). 2021 May 22;12(6):598. doi: 10.3390/mi12060598. Micromachines (Basel). 2021. PMID: 34067351 Free PMC article.
-
An Interface Heterostructure of NiO and CeO2 for Using Electrolytes of Low-Temperature Solid Oxide Fuel Cells.Nanomaterials (Basel). 2021 Aug 5;11(8):2004. doi: 10.3390/nano11082004. Nanomaterials (Basel). 2021. PMID: 34443835 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

