Lanthanide complexes combined with chiral salen ligands: application in the enantioselective epoxidation reaction of α,β-unsaturated ketones
- PMID: 35519594
- PMCID: PMC9063913
- DOI: 10.1039/c9ra01529a
Lanthanide complexes combined with chiral salen ligands: application in the enantioselective epoxidation reaction of α,β-unsaturated ketones
Abstract
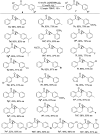
Readily available lanthanide amides Ln[N(SiMe3)2]3 (Ln = Nd (1), Sm (2), Eu (3), Yb (4), La (5)), combined with chiral salen ligands H2La ((S,S)-N,N'-di-(3,5-disubstituted-salicylidene)-1,2-cyclohexanediamine) and H2Lb ((S,S)-N,N'-di-(3,5-disubstituted-salicylidene)-1,2-diphenyl-1,2-ethanediamine) were employed in the enantioselective epoxidation of α,β-unsaturated ketones. It was found that the salen-La complex shows the highest efficiency and enantioselectivity. A relatively broad scope of α,β-unsaturated ketones was investigated, and excellent yields (up to 99%) and moderate to good enantioselectivities (37-87%) of the target molecules were achieved.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
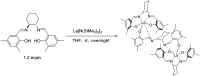

Similar articles
-
Enantioselective Michael Addition of Malononitrile to Unsaturated Ketones Catalyzed by Rare-Earth Metal Amides RE[N(SiMe3)2]3 with Phenoxy-Functionalized TsDPEN Ligands.J Org Chem. 2023 Sep 15;88(18):13205-13213. doi: 10.1021/acs.joc.3c01435. Epub 2023 Sep 6. J Org Chem. 2023. PMID: 37672624
-
Systematic study on the structures of salen type lanthanide complexes tuned by lanthanide contraction and corresponding luminescence.Dalton Trans. 2013 Jul 14;42(26):9482-9. doi: 10.1039/c3dt50534k. Epub 2013 May 10. Dalton Trans. 2013. PMID: 23660709
-
Near-infrared luminescence and RNA cleavage ability of lanthanide Schiff base complexes derived from N,N'-bis(3-methoxysalicylidene)ethylene-1,2-diamine ligands.J Inorg Biochem. 2016 Oct;163:194-205. doi: 10.1016/j.jinorgbio.2016.07.017. Epub 2016 Aug 5. J Inorg Biochem. 2016. PMID: 27554192
-
Asymmetric epoxidation of unsaturated ketones catalyzed by heterobimetallic rare earth-lithium complexes bearing phenoxy-functionalized chiral diphenylprolinolate ligand.Org Lett. 2014 Sep 5;16(17):4516-9. doi: 10.1021/ol5020398. Epub 2014 Aug 22. Org Lett. 2014. PMID: 25148072
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
References
-
- Kitamura M. Suga S. Kawai K. Noyori R. J. Am. Chem. Soc. 1986;108:6071–6072. doi: 10.1021/ja00279a083. - DOI - PubMed
- Noyori R. Kitamura M. Angew. Chem., Int. Ed. Engl. 1991;30:49–69. doi: 10.1002/anie.199100491. - DOI
- Quirmbach M. Kless A. Holz J. Tararov B. Börner A. Tetrahedron: Asymmetry. 1999;10:1803–1811. doi: 10.1016/S0957-4166(99)00165-2. - DOI
- Kless A. Kadyrov R. Börner A. Holz J. Kagan H. B. Tetrahedron Lett. 1995;36:4601–4602. doi: 10.1016/0040-4039(95)00844-3. - DOI
- Dimauro E. F. Kozlowski M. C. Org. Lett. 2001;3(19):3053–3056. doi: 10.1021/ol016535u. - DOI - PubMed
-
- DiMauro E. F. Kozlowski M. C. J. Am. Chem. Soc. 2002;124:12668–12670. doi: 10.1021/ja026498h. - DOI - PubMed
- Garcfa C. LaRochelle L. K. Walsh P. J. J. Am. Chem. Soc. 2002;124:10970–10971. doi: 10.1021/ja026568k. - DOI - PubMed
- Belokon Y. N. Green B. Ikonnikov N. S. North M. Tararov V. I. Tetrahedron Lett. 1999;40:8147–8149. doi: 10.1016/S0040-4039(99)01677-9. - DOI
- Yabu K. Masumoto S. Kanai M. Curran D. P. Shibasaki M. Tetrahedron Lett. 2002;43:2923–2925. doi: 10.1016/S0040-4039(02)00451-3. - DOI
- Masumoto S. Suzuki M. Kanai M. Shibasaki M. Tetrahedron Lett. 2002;43:8647–8650. doi: 10.1016/S0040-4039(02)02135-4. - DOI
- Tian S. K. Deng L. J. Am. Chem. Soc. 2001;123:6195–6197. doi: 10.1021/ja010690m. - DOI - PubMed
- Deng H. Snapper M. P. Hoveyda A. H. Angew. Chem., Int. Ed. 2002;41:1009–1011. doi: 10.1002/1521-3773(20020315)41:6<1009::AID-ANIE1009>3.0.CO;2-F. - DOI - PubMed
- Chen F. X. Feng X. M. Qin B. Zhang G. L. Jiang Y. Z. Org. Lett. 2003;5(6):949–952. doi: 10.1021/ol034158a. - DOI - PubMed
-
- Evans D. A. Burgey C. S. Paras N. A. Vojkovsky T. Tregay S. W. J. Am. Chem. Soc. 1998;120:5824–5825. doi: 10.1021/ja980549m. - DOI
- Evans D. A. Tregay S. W. Burgey C. S. Paras N. A. Vojkovsky T. J. Am. Chem. Soc. 2000;122:7936–7943. doi: 10.1021/ja000913t. - DOI
- Yuan Y. Zhang X. Ding K. Angew. Chem., Int. Ed. 2003;42:5478–5480. doi: 10.1002/anie.200352535. - DOI - PubMed
- Evans D. A. Wu J. J. Am. Chem. Soc. 2005;127:8006–8007. doi: 10.1021/ja0522130. - DOI - PubMed
- Huston G. E. Dave A. H. Rawal V. H. Org. Lett. 2007;9:3869–3872. doi: 10.1021/ol071342d. - DOI - PubMed
-
- Denes F. D. Perez-Luna A. Chemla F. Chem. Rev. 2010;110:2366–2368. doi: 10.1021/cr800420x. - DOI - PubMed
- Corkey B. K. Toste F. D. J. Am. Chem. Soc. 2005;125:17168–17169. doi: 10.1021/ja055059q. - DOI - PubMed
- Yang T. Ferrali A. Sladojevich F. Campbell L. Dixon D. J. Am. Chem. Soc. 2009;131:9140–9141. doi: 10.1021/ja9004859. - DOI - PubMed
- Matsuzawa A. Mashiko T. Kumagai N. Shibasaki M. Angew. Chem., Int. Ed. 2011;50:7616–7618. doi: 10.1002/anie.201102114. - DOI - PubMed
- Shaw S. White J. D. J. Am. Chem. Soc. 2014;136:13578–13581. doi: 10.1021/ja507853f. - DOI - PubMed
LinkOut - more resources
Full Text Sources

