Engineering Vascularized Islet Macroencapsulation Devices: An in vitro Platform to Study Oxygen Transport in Perfused Immobilized Pancreatic Beta Cell Cultures
- PMID: 35519615
- PMCID: PMC9061948
- DOI: 10.3389/fbioe.2022.884071
Engineering Vascularized Islet Macroencapsulation Devices: An in vitro Platform to Study Oxygen Transport in Perfused Immobilized Pancreatic Beta Cell Cultures
Abstract
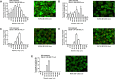
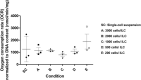
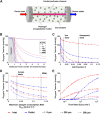
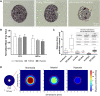
Islet encapsulation devices serve to deliver pancreatic beta cells to type 1 diabetic patients without the need for chronic immunosuppression. However, clinical translation is hampered by mass transport limitations causing graft hypoxia. This is exacerbated in devices relying only on passive diffusion for oxygenation. Here, we describe the application of a cylindrical in vitro perfusion system to study oxygen effects on islet-like clusters immobilized in alginate hydrogel. Mouse insulinoma 6 islet-like clusters were generated using microwell plates and characterized with respect to size distribution, viability, and oxygen consumption rate to determine an appropriate seeding density for perfusion studies. Immobilized clusters were perfused through a central channel at different oxygen tensions. Analysis of histological staining indicated the distribution of viable clusters was severely limited to near the perfusion channel at low oxygen tensions, while the distribution was broadest at normoxia. The results agreed with a 3D computational model designed to simulate the oxygen distribution within the perfusion device. Further simulations were generated to predict device performance with human islets under in vitro and in vivo conditions. The combination of experimental and computational findings suggest that a multichannel perfusion strategy could support in vivo viability and function of a therapeutic islet dose.
Keywords: artificial vascularization; beta cells; encapsulation; immobilized culture; oxygen mass transport; oxygen model; type 1 diabetes.
Copyright © 2022 S. A., K. S., L., M., S., R. L. and C. A..
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
An in vitro Perfused Macroencapsulation Device to Study Hemocompatibility and Survival of Islet-Like Cell Clusters.Front Bioeng Biotechnol. 2021 May 28;9:674125. doi: 10.3389/fbioe.2021.674125. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34124024 Free PMC article.
-
In vivovascularization and islet function in a microwell device for pancreatic islet transplantation.Biomed Mater. 2021 Apr 21;16(3). doi: 10.1088/1748-605X/abf5ec. Biomed Mater. 2021. PMID: 33831849
-
In situ type I oligomeric collagen macroencapsulation promotes islet longevity and function in vitro and in vivo.Am J Physiol Endocrinol Metab. 2018 Oct 1;315(4):E650-E661. doi: 10.1152/ajpendo.00073.2018. Epub 2018 Jun 12. Am J Physiol Endocrinol Metab. 2018. PMID: 29894201 Free PMC article.
-
Oxygenation strategies for encapsulated islet and beta cell transplants.Adv Drug Deliv Rev. 2019 Jan 15;139:139-156. doi: 10.1016/j.addr.2019.05.002. Epub 2019 May 8. Adv Drug Deliv Rev. 2019. PMID: 31077781 Review.
-
Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: Cells, biomaterials, and devices.Biotechnol Bioeng. 2016 Jul;113(7):1381-402. doi: 10.1002/bit.25895. Epub 2016 Jan 4. Biotechnol Bioeng. 2016. PMID: 26615050 Free PMC article. Review.
Cited by
-
Oxygenation and function of endocrine bioartificial pancreatic tissue constructs under flow for preclinical optimization.J Tissue Eng. 2025 Jan 23;16:20417314241284826. doi: 10.1177/20417314241284826. eCollection 2025 Jan-Dec. J Tissue Eng. 2025. PMID: 39866963 Free PMC article.
-
Long-term cultures of human pancreatic islets in self-assembling peptides hydrogels.Front Bioeng Biotechnol. 2023 Feb 23;11:1105157. doi: 10.3389/fbioe.2023.1105157. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36911193 Free PMC article.
-
In vitro oxygen imaging of acellular and cell-loaded beta cell replacement devices.Sci Rep. 2023 Sep 20;13(1):15641. doi: 10.1038/s41598-023-42099-w. Sci Rep. 2023. PMID: 37730815 Free PMC article.
-
Islet cell spheroids produced by a thermally sensitive scaffold: a new diabetes treatment.J Nanobiotechnology. 2024 Oct 26;22(1):657. doi: 10.1186/s12951-024-02891-w. J Nanobiotechnology. 2024. PMID: 39456025 Free PMC article.
-
Hypoxia within subcutaneously implanted macroencapsulation devices limits the viability and functionality of densely loaded islets.Front Transplant. 2023 Nov 17;2:1257029. doi: 10.3389/frtra.2023.1257029. eCollection 2023. Front Transplant. 2023. PMID: 38993891 Free PMC article.
References
-
- Avgoustiniatos E. S., Dionne D. F., Yarmush M. L., Colton C. K. (2007). Measurements of the Effective Diffusion Coefficient of Oxygen in Pancreatic Islets. Ind. Eng. Chem. Res. 46 (19), 6157–6163. 10.1021/ie070662y - DOI
LinkOut - more resources
Full Text Sources

