Glucagon-Like Peptide 1 Receptor Agonist Improves Renal Tubular Damage in Mice with Diabetic Kidney Disease
- PMID: 35519661
- PMCID: PMC9064072
- DOI: 10.2147/DMSO.S353717
Glucagon-Like Peptide 1 Receptor Agonist Improves Renal Tubular Damage in Mice with Diabetic Kidney Disease
Abstract
Purpose: This study aims to investigate the renal protective effect of glucagon-like peptide 1 receptor agonist (GLP-1RA) on improving renal tubular damage in diabetic kidney disease (DKD) and to explore the potential mechanism of GLP-1RA on renal tubular protection.
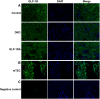
Methods: Long-acting GLP-1RA was used to treat DKD mice for 12 weeks. The label-free quantitative proteomic analysis of renal proteins was conducted to explore the differentially expressed proteins (DEPs) in the renal tissues of the control, DKD and GLP-1RA groups. The DEPs and markers of renal tubular injury were verified by qPCR in vivo and in vitro. The expression of glucagon-likepeptide-1 receptor (GLP-1R) in renal tubules was determined by immunofluorescence staining.
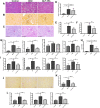
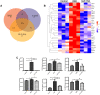
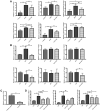
Results: GLP-1RA treatment significantly improved the tubular damages in kidney tissues of DKD mice and mTEC cells stimulated by high glucose (HG). Proteomics analysis revealed that 30 proteins in kidney tissue were differentially expressed among three groups. Seminal vesicle secretory protein 6 (SVS6) was the most differentially expressed protein in kidney tissues among three groups of mice. The expression changes of Svs6 mRNA in vitro and in vivo detected by qPCR were consistent with the results of proteomic analysis. Furthermore, reduction of Svs6 expression by SVS6 siRNA could attenuate HG-stimulated tubular injury in mTEC cells. Immunofluorescence staining also found that GLP-1R was widely expressed in renal tubules in vitro and in vivo.
Conclusion: GLP-1RA significantly improved renal tubular damage in DKD mice. SVS6 may be a potential therapeutic target for GLP-1RA in the treatment of DKD.
Keywords: diabetic kidney disease; glucagon-like peptide-1 receptor agonist; renal tubular injury; seminal vesicle secretory protein 6.
© 2022 Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest for this work.
Figures

References
-
- Shaheen FA, Al-Khader AA. Epidemiology and causes of end stage renal disease (ESRD). Saudi J Kidney Dis Transpl. 2005;16(3):277–281. - PubMed
-
- Han J, Pang X, Shi X, Zhang Y, Peng Z, Xing Y. Ginkgo biloba extract EGB761 ameliorates the extracellular matrix accumulation and mesenchymal transformation of renal tubules in diabetic kidney disease by inhibiting endoplasmic reticulum stress. Biomed Res Int. 2021;2021:6657206. doi:10.1155/2021/6657206 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

