Hydrogels as artificial matrices for cell seeding in microfluidic devices
- PMID: 35519701
- PMCID: PMC9058401
- DOI: 10.1039/d0ra08566a
Hydrogels as artificial matrices for cell seeding in microfluidic devices
Abstract
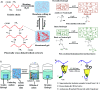
Hydrogel-based artificial scaffolds play a vital role in shifting in vitro models from two-dimensional (2D) cell culture to three-dimensional (3D) cell culture. Microfluidic 3D cell culture systems with a hydrogel matrix encourage biomedical researchers to replace in vivo models with 3D in vitro models with a cellular microenvironment that resembles physiological conditions with greater fidelity. Hydrogels can be designed as an artificial extracellular matrix scaffold for providing spatial orientation and promoting cellular interactions with surroundings. Selecting the appropriate hydrogels and their fabrication techniques are the key to mimic the in vivo mechanical environment. Moreover, combining a microfluidic technique with a hydrogel-based 3D cell culture system can create a complex and controlled microenvironment for the cells by placing small biosamples inside the microchannel. This paper provides an overview of the structural similarities of the hydrogels as an extracellular matrix (ECM), their classification and fabrication techniques as an ECM, and their use in microfluidic 3D cell culture systems. Finally, the paper presents the current challenges and future perspectives of using hydrogel scaffolds in microfluidic 3D cell culture systems.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Nothing to declare.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources

