In vitro kinetic release study, antimicrobial activity and in vivo toxicity profile of a kojic acid ester-based nanoemulsion for topical application
- PMID: 35519703
- PMCID: PMC9058481
- DOI: 10.1039/d0ra04807k
In vitro kinetic release study, antimicrobial activity and in vivo toxicity profile of a kojic acid ester-based nanoemulsion for topical application
Abstract
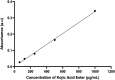
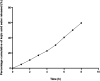
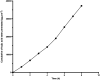
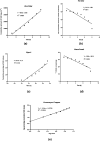
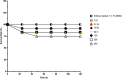
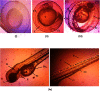
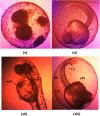
Nanoemulsions have emerged as novel vehicles for drug delivery that allow sustained or controlled release for topical application. In this study, kojic acid ester-based nanoemulsion (KAE-NA) was analyzed for in vitro permeation evaluation, kinetic release study, in vitro antimicrobial activity and in vivo toxicity profile on embryonic zebrafish (Danio rerio). Based on KAE-NA in vitro permeation evaluation, the percentage of permeation was significantly improved from 4.94% at 1 h to 59.64% at 8 h of application. The permeation rate of KAE-NA at 8 h was 4659.50 μg cm-2 h-1 (initial concentration, C 0 = 2000 μg mL-1) with a permeability coefficient (K p) value of 0.48 cm h-1. The kinetic release analysis showed the Korsmeyer-Peppas model was the best fitted kinetic model with high linearity [R 2 = 0.9964]. Antimicrobial activity of KAE-NA was studied against the skin pathogen bacteria Staphylococcus aureus ATCC 43300. The results indicated that the inhibition zone size of the KAE-NA (8.00 ± 0.0 mm) was slightly bigger than that of its active ingredient, kojic acid ester (6.5 ± 0.0 mm). The toxicity profile of KAE-NA on embryonic zebrafish revealed less toxicity with LC50 (50% lethal concentration) more than 500 μg mL-1. The survival rate of the embryonic zebrafish was more than 80% when treated at doses ranging from 7.81-250 μg mL-1 and showed normal development throughout the experiment without any observed deformation. Hence, KAE-NA proved to be less toxic on the embryonic zebrafish.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Duarah S. Pujari K. Durai R. D. Narayanan V. H. B. Int. J. Appl. Pharm. 2016;8(1):8–12.
-
- Dasguptaa N. Ranjanab S. Mundraa S. Ramalingama C. Kumarc A. Int. J. Food Prop. 2015:1–21.
-
- Samson S. Basri M. Masoumi H. R. F. Karjiban R. A. Malek E. A. RSC Adv. 2016;6:17845–17856. doi: 10.1039/C5RA24379C. - DOI
-
- Sharma S. Silva J. Abebe W. Sousa S. M. Duarte V. G. Machado M. I. L. Sarangdevot K. RSC Adv. 2012;1(3):408–415.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

